Cdc25B cooperates with Cdc25A to induce mitosis but has a unique role in activating cyclin B1-Cdk1 at the centrosome
- PMID: 16216921
- PMCID: PMC2171226
- DOI: 10.1083/jcb.200503066
Cdc25B cooperates with Cdc25A to induce mitosis but has a unique role in activating cyclin B1-Cdk1 at the centrosome
Abstract
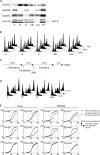
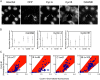
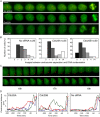
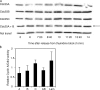
Cdc25 phosphatases are essential for the activation of mitotic cyclin-Cdks, but the precise roles of the three mammalian isoforms (A, B, and C) are unclear. Using RNA interference to reduce the expression of each Cdc25 isoform in HeLa and HEK293 cells, we observed that Cdc25A and -B are both needed for mitotic entry, whereas Cdc25C alone cannot induce mitosis. We found that the G2 delay caused by small interfering RNA to Cdc25A or -B was accompanied by reduced activities of both cyclin B1-Cdk1 and cyclin A-Cdk2 complexes and a delayed accumulation of cyclin B1 protein. Further, three-dimensional time-lapse microscopy and quantification of Cdk1 phosphorylation versus cyclin B1 levels in individual cells revealed that Cdc25A and -B exert specific functions in the initiation of mitosis: Cdc25A may play a role in chromatin condensation, whereas Cdc25B specifically activates cyclin B1-Cdk1 on centrosomes.
Figures


References
-
- Bernardi, R., D.A. Liebermann, and B. Hoffman. 2000. Cdc25A stability is controlled by the ubiquitin-proteasome pathway during cell cycle progression and terminal differentiation. Oncogene. 19:2447–2454. - PubMed
-
- Bulavin, D.V., Y. Higashimoto, Z.N. Demidenko, S. Meek, P. Graves, C. Phillips, H. Zhao, S.A. Moody, E. Appella, H. Piwnica-Worms, and A.J. Fornace. 2003. Dual phosphorylation controls Cdc25 phosphatases and mitotic entry. Nat. Cell Biol. 5:545–551. - PubMed
-
- Chae, H.D., J. Yun, Y.J. Bang, and D.Y. Shin. 2004. Cdk2-dependent phosphorylation of the NF-Y transcription factor is essential for the expression of the cell cycle-regulatory genes and cell cycle G1/S and G2/M transitions. Oncogene. 23:4084–4088. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

