Integrin-dependent actomyosin contraction regulates epithelial cell scattering
- PMID: 16216928
- PMCID: PMC2171213
- DOI: 10.1083/jcb.200506152
Integrin-dependent actomyosin contraction regulates epithelial cell scattering
Abstract
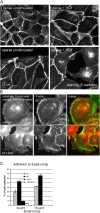
The scattering of Madin-Darby canine kidney cells in vitro mimics key aspects of epithelial-mesenchymal transitions during development, carcinoma cell invasion, and metastasis. Scattering is induced by hepatocyte growth factor (HGF) and is thought to involve disruption of cadherin-dependent cell-cell junctions. Scattering is enhanced on collagen and fibronectin, as compared with laminin1, suggesting possible cross talk between integrins and cell-cell junctions. We show that HGF does not trigger any detectable decrease in E-cadherin function, but increases integrin-mediated adhesion. Time-lapse imaging suggests that tension on cell-cell junctions may disrupt cell-cell adhesion. Varying the density and type of extracellular matrix proteins shows that scattering correlates with stronger integrin adhesion and increased phosphorylation of the myosin regulatory light chain. To directly test the role of integrin-dependent traction forces, substrate compliance was varied. Rigid substrates that produce high traction forces promoted scattering, in comparison to more compliant substrates. We conclude that integrin-dependent actomyosin traction force mediates the disruption of cell-cell adhesion during epithelial cell scattering.
Figures






References
-
- Avizienyte, E., A.W. Wyke, R.J. Jones, G.W. McLean, M.A. Westhoff, V.G. Brunton, and M.C. Frame. 2002. Src-induced de-regulation of E-cadherin in colon cancer cells requires integrin signalling. Nat. Cell Biol. 4:632–638. - PubMed
-
- Balkovetz, D.F., and V. Sambandam. 1999. Dynamics of E-cadherin and gamma-catenin complexes during dedifferentiation of polarized MDCK cells. Kidney Int. 56:910–921. - PubMed

