Dynamics of the acetylcholine receptor pore at the gating transition state
- PMID: 16217024
- PMCID: PMC1257706
- DOI: 10.1073/pnas.0505090102
Dynamics of the acetylcholine receptor pore at the gating transition state
Abstract
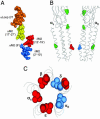
Neuromuscular acetylcholine receptors (AChRs) are ion channels that alternatively adopt stable conformations that either allow (open) or prohibit (closed) ionic conduction. We probed the dynamics of pore (M2) residues at the diliganded gating transition state by using single-channel kinetic and rate-equilibrium free energy relationship (phi-value) analyses of mutant AChRs. The mutations were at the equatorial (9') position of the alpha, beta, and epsilon subunits (n = 15) or at sites between the equator and the extracellular domain in the alpha-subunit (n = 8). We also studied AChRs having only one of the two alpha-subunits mutated. The results indicate that the alpha-subunit, like the delta-subunit, has a region of flexure near the middle of M2, that the two alpha-subunits experience distinct energy barriers to gating at the equator (but not elsewhere), and that the collective subunit motions at the equator are asymmetric during the AChR gating isomerization.
Figures


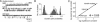

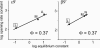
References
-
- Auerbach, A. (2003) Sci. STKE 2003, re11. - PubMed
-
- Karlin, A. (2002) Nat. Rev. Neurosci. 3, 102–114. - PubMed
-
- Changeux, J. P. & Edelstein, S. J. (1998) Neuron 21, 959–980. - PubMed
-
- Tobimatsu, T., Fujita, Y., Fukuda, K., Tanaka, K., Mori, Y., Konno, T., Mishina, M. & Numa, S. (1987) FEBS Lett. 222, 56–62. - PubMed
-
- Miyazawa, A., Fujiyoshi, Y. & Unwin, N. (2003) Nature 423, 949–955. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

