Severe muscle disease-causing desmin mutations interfere with in vitro filament assembly at distinct stages
- PMID: 16217025
- PMCID: PMC1250230
- DOI: 10.1073/pnas.0504568102
Severe muscle disease-causing desmin mutations interfere with in vitro filament assembly at distinct stages
Abstract
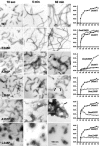
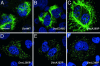
Desmin is the major intermediate filament (IF) protein of muscle. Recently, mutations of the desmin gene have been reported to cause familial or sporadic forms of human skeletal, as well as cardiac, myopathy, termed desmin-related myopathy (DRM). The impact of any of these mutations on filament assembly and integration into the cytoskeletal network of myocytes is currently not understood, despite the fact that all cause the same histopathological defect, i.e., desmin aggregation. To gain more insight into the molecular basis of this process, we investigated how mutations within the alpha-helical rod domain of desmin affect both the assembly of the recombinant protein in vitro as well as the filament-forming capacity in cDNA-transfected cells. Whereas 6 of 14 mutants assemble into seemingly normal IFs in the test tube, the other mutants interfere with the assembly process at distinct stages, i.e., tetramer formation, unit-length filament (ULF) formation, filament elongation, and IF maturation. Correspondingly, the mutants with in vitro assembly defects yield dot-like aggregates in transfected cells, whereas the mutants that form IFs constitute a seemingly normal IF cytoskeleton in the cellular context. At present, it is entirely unclear why the latter mutant proteins also lead to aggregate formation in myocytes. Hence, these findings may be a starting point to dissect the contribution of the individual subdomains for desmin pathology and, eventually, the development of therapeutic interventions.
Figures
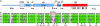

References
-
- Herrmann, H. & Aebi, U. (2004) Annu. Rev. Biochem. 73, 749–789. - PubMed
-
- Bär, H., Strelkov, S. V., Sjoberg, G., Aebi, U. & Herrmann, H. (2004) J. Struct. Biol. 148, 137–152. - PubMed
-
- Bennardini, F., Wrzosek, A. & Chiesi, M. (1992) Circ. Res. 71, 288–294. - PubMed
-
- Omary, M. B., Coulombe, P. A. & McLean, W. H. (2004) N. Engl. J. Med. 351, 2087–2100. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

