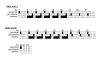Randomized phase II--study evaluating EGFR targeting therapy with cetuximab in combination with radiotherapy and chemotherapy for patients with locally advanced pancreatic cancer--PARC: study protocol [ISRCTN56652283]
- PMID: 16219105
- PMCID: PMC1266352
- DOI: 10.1186/1471-2407-5-131
Randomized phase II--study evaluating EGFR targeting therapy with cetuximab in combination with radiotherapy and chemotherapy for patients with locally advanced pancreatic cancer--PARC: study protocol [ISRCTN56652283]
Abstract
Background: Pancreatic cancer is the fourth commonest cause of death from cancer in men and women. Advantages in surgical techniques, radiation therapy techniques, chemotherapeutic regimes, and different combined-modality approaches have yielded only a modest impact on the prognosis of patients with pancreatic cancer. Thus there is clearly a need for additional strategies. One approach involves using the identification of a number of molecular targets that may be responsible for the resistance of cancer cells to radiation or to other cytotoxic agents. As such, these molecular determinants may serve as targets for augmentation of the radiotherapy or chemotherapy response. Of these, the epidermal growth factor receptor (EGFR) has been a molecular target of considerable interest and investigation, and there has been a tremendous surge of interest in pursuing targeted therapy of cancers via inhibition of the EGFR.
Methods/design: The PARC study is designed as an open, controlled, prospective, randomized phase II trial. Patients in study arm A will be treated with chemoradiation using intensity modulated radiation therapy (IMRT) combined with gemcitabine and simultaneous cetuximab infusions. After chemoradiation the patients receive gemcitabine infusions weekly over 4 weeks. Patients in study arm B will be treated with chemoradiation using intensity modulated radiation therapy (IMRT) combined with gemcitabine and simultaneous cetuximab infusions. After chemoradiation the patients receive gemcitabine weekly over 4 weeks and cetuximab infusions over 12 weeks. A total of 66 patients with locally advanced adenocarcinoma of the pancreas will be enrolled. An interim analysis for patient safety reasons will be done one year after start of recruitment. Evaluation of the primary endpoint will be performed two years after the last patient's enrollment.
Discussion: The primary objective of this study is to evaluate the feasibility and the toxicity profile of trimodal therapy in pancreatic adenocarcinoma with chemoradiation therapy with gemcitabine and intensity modulated radiation therapy (IMRT) and EGFR-targeted therapy using cetuximab and to compare between two different methods of cetuximab treatment schedules (concomitant versus concomitant and sequential cetuximab treatment). Secondary objectives are to determine the role and the mechanism of cetuximab in patient's chemoradiation regimen, the response rate, the potential of this combined modality treatment to concert locally advanced lesions to potentially resectable lesions, the time to progression interval and the quality of life.
References
-
- Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. - PubMed
-
- Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, Beger H, Fernandez-Cruz L, Dervenis C, Lacaine F, Falconi M, Pederzoli P, Pap A, Spooner D, Kerr DJ, Buchler MW. European Study Group for Pancreatic Cancer. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–10. doi: 10.1056/NEJMoa032295. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous