Respiratory epithelial cells require Toll-like receptor 4 for induction of human beta-defensin 2 by lipopolysaccharide
- PMID: 16219107
- PMCID: PMC1276817
- DOI: 10.1186/1465-9921-6-116
Respiratory epithelial cells require Toll-like receptor 4 for induction of human beta-defensin 2 by lipopolysaccharide
Abstract
Background: The respiratory epithelium is a major portal of entry for pathogens and employs innate defense mechanisms to prevent colonization and infection. Induced expression of human beta-defensin 2 (HBD2) represents a direct response by the epithelium to potential infection. Here we provide evidence for the critical role of Toll-like receptor 4 (TLR4) in lipopolysaccharide (LPS)-induced HBD2 expression by human A549 epithelial cells.
Methods: Using RTPCR, fluorescence microscopy, ELISA and luciferase reporter gene assays we quantified interleukin-8, TLR4 and HBD2 expression in unstimulated or agonist-treated A549 and/or HEK293 cells. We also assessed the effect of over expressing wild type and/or mutant TLR4, MyD88 and/or Mal transgenes on LPS-induced HBD2 expression in these cells.
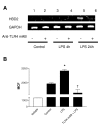
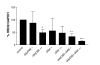
Results: We demonstrate that A549 cells express TLR4 on their surface and respond directly to Pseudomonas LPS with increased HBD2 gene and protein expression. These effects are blocked by a TLR4 neutralizing antibody or functionally inactive TLR4, MyD88 and/or Mal transgenes. We further implicate TLR4 in LPS-induced HBD2 production by demonstrating HBD2 expression in LPS non-responsive HEK293 cells transfected with a TLR4 expression plasmid.
Conclusion: This data defines an additional role for TLR4 in the host defense in the lung.
Figures


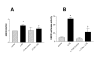
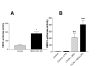
Similar articles
-
[Expression of toll-like receptor 4 in human alveolar epithelial cells and its role in cellular inflammation].Zhonghua Yi Xue Za Zhi. 2008 Aug 5;88(30):2112-6. Zhonghua Yi Xue Za Zhi. 2008. PMID: 19080471 Chinese.
-
Bacterial lipopolysaccharide induces increased expression of toll-like receptor (TLR) 4 and downstream TLR signaling molecules in bovine mammary epithelial cells.Vet Res. 2008 Mar-Apr;39(2):11. doi: 10.1051/vetres:2007047. Epub 2007 Dec 21. Vet Res. 2008. PMID: 18096120
-
[Influence of lipopolysaccharide on the expression of Toll-like receptor 4 in human bronchial epithelium cell in patients with chronic obstructive pulmonary disease].Zhonghua Jie He He Hu Xi Za Zhi. 2006 Jul;29(7):448-51. Zhonghua Jie He He Hu Xi Za Zhi. 2006. PMID: 17045043 Chinese.
-
Airway epithelia regulate expression of human beta-defensin 2 through Toll-like receptor 2.FASEB J. 2003 Sep;17(12):1727-9. doi: 10.1096/fj.02-0616fje. Epub 2003 Jul 3. FASEB J. 2003. PMID: 12958190
-
Human β-defensin 2: a connection between infections and allergic skin diseases.Acta Dermatovenerol Alp Pannonica Adriat. 2024 Sep;33(3):135-139. Acta Dermatovenerol Alp Pannonica Adriat. 2024. PMID: 39324351 Review.
Cited by
-
Inhibition of NFkappaB by the natural product Withaferin A in cellular models of Cystic Fibrosis inflammation.J Inflamm (Lond). 2009 May 13;6:15. doi: 10.1186/1476-9255-6-15. J Inflamm (Lond). 2009. PMID: 19439083 Free PMC article.
-
Expression and functional analysis of Toll-like receptor 4 in human cervical carcinoma.J Membr Biol. 2014 Jul;247(7):591-9. doi: 10.1007/s00232-014-9675-7. Epub 2014 May 31. J Membr Biol. 2014. PMID: 24878539
-
Human beta defensin-2 protects the epithelial barrier during methicillin-resistant Staphylococcus aureus infection in chronic rhinosinusitis with nasal polyps.Front Cell Infect Microbiol. 2025 May 9;15:1551080. doi: 10.3389/fcimb.2025.1551080. eCollection 2025. Front Cell Infect Microbiol. 2025. PMID: 40415954 Free PMC article.
-
β-Defensin-2 is overexpressed in human vocal cord polyps.Eur Arch Otorhinolaryngol. 2017 Feb;274(2):901-907. doi: 10.1007/s00405-016-4270-4. Epub 2016 Sep 1. Eur Arch Otorhinolaryngol. 2017. PMID: 27586391
-
Antimicrobial proteins and polypeptides in pulmonary innate defence.Respir Res. 2006 Feb 17;7(1):29. doi: 10.1186/1465-9921-7-29. Respir Res. 2006. PMID: 16503962 Free PMC article. Review.
References
-
- Bowie A, O'Neill LA. The interleukin-1 receptor/Toll-like receptor superfamily: signal generators for pro-inflammatory interleukins and microbial products. J Leukoc Biol. 2000;67:508–514. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

