Genetic evidence that nonhomologous disjunction and meiotic drive are properties of wild-type Drosophila melanogaster male meiosis
- PMID: 16219792
- PMCID: PMC1456159
- DOI: 10.1534/genetics.104.036806
Genetic evidence that nonhomologous disjunction and meiotic drive are properties of wild-type Drosophila melanogaster male meiosis
Abstract
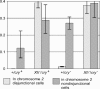
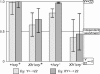
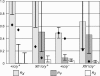
We have followed sex and second chromosome disjunction, and the effects of these chromosomes on sperm function, in four genotypes: wild-type males, males deficient for the Y-linked crystal locus, males with an X chromosome heterochromatic deficiency that deletes all X-Y pairing sites, and males with both deficiencies. Both mutant situations provoke chromosome misbehavior, but the disjunctional defects are quite different. Deficiency of the X heterochromatin, consonant with the lack of pairing sites, mostly disrupts X-Y disjunction with a decidedly second-level effect on major autosome behavior. Deleting crystal, consonant with the cytological picture of postpairing chromatin-condensation problems, disrupts sex and autosome disjunction equally. Even when the mutant-induced nondisjunction has very different mechanics, however, and even more importantly, even in the wild type, there is strong, and similar, meiotic drive. The presence of meiotic drive when disjunction is disrupted by distinctly different mechanisms supports the notion that drive is a normal cellular response to meiotic problems rather than a direct effect of particular mutants. Most surprisingly, in both wild-type and crystal-deficient males the Y chromosome moves to the opposite pole from a pair of nondisjoined second chromosomes nearly 100% of the time. This nonhomologous interaction is, however, absent when the X heterochromatin is deleted. The nonhomologous disjunction of the sex and second chromosomes may be the genetic consequence of the chromosomal compartmentalization seen by deconvolution microscopy, and the absence of Y-2 disjunction when the X heterochromatin is deleted suggests that XY pairing itself, or a previously unrecognized heterochromatic function, is prerequisite to this macrostructural organization of the chromosomes.
Figures


Similar articles
-
Genetic analysis of sex chromosomal meiotic mutants in Drosophilia melanogaster.Genetics. 1972 Jun;71(2):255-86. doi: 10.1093/genetics/71.2.255. Genetics. 1972. PMID: 4625747 Free PMC article.
-
On the roles of heterochromatin and euchromatin in meiosis in drosophila: mapping chromosomal pairing sites and testing candidate mutations for effects on X-Y nondisjunction and meiotic drive in male meiosis.Genetica. 2000;109(1-2):77-93. doi: 10.1023/a:1026536200594. Genetica. 2000. PMID: 11293799 Review.
-
Meiosis in male Drosophila melanogaster I. Isolation and characterization of meiotic mutants affecting second chromosome disjuction.Genetics. 1974 Dec;78(4):1127-42. doi: 10.1093/genetics/78.4.1127. Genetics. 1974. PMID: 4376098 Free PMC article.
-
Male sterility and meiotic drive associated with sex chromosome rearrangements in Drosophila. Role of X-Y pairing.Genetics. 1998 May;149(1):143-55. doi: 10.1093/genetics/149.1.143. Genetics. 1998. PMID: 9584092 Free PMC article.
-
The license to pair: identification of meiotic pairing sites in Drosophila.Chromosoma. 1996 Sep;105(3):135-41. doi: 10.1007/BF02509494. Chromosoma. 1996. PMID: 8781181 Review.
Cited by
-
The cellular basis of hybrid dysgenesis and Stellate regulation in Drosophila.Curr Opin Genet Dev. 2015 Oct;34:88-94. doi: 10.1016/j.gde.2015.09.003. Epub 2015 Oct 24. Curr Opin Genet Dev. 2015. PMID: 26451497 Free PMC article. Review.
-
Synaptonemal Complex-Deficient Drosophila melanogaster Females Exhibit Rare DSB Repair Events, Recurrent Copy-Number Variation, and an Increased Rate of de Novo Transposable Element Movement.G3 (Bethesda). 2020 Feb 6;10(2):525-537. doi: 10.1534/g3.119.400853. G3 (Bethesda). 2020. PMID: 31882405 Free PMC article.
-
Repeat-associated siRNAs cause chromatin silencing of retrotransposons in the Drosophila melanogaster germline.Nucleic Acids Res. 2007;35(16):5430-8. doi: 10.1093/nar/gkm576. Epub 2007 Aug 15. Nucleic Acids Res. 2007. PMID: 17702759 Free PMC article.
References
-
- Bozzetti, M. P., S. Massari, P. Finelli, F. Meggio, L. A. Pinna et al., 1995. The Ste locus, a component of the parasitic cry-Ste system of Drosophila melanogaster, encodes a protein that forms crystals in primary spermatocytes and mimics properties of the beta subunit of casein kinase 2. Proc. Natl. Acad. Sci. USA 92: 6067–6071. - PMC - PubMed
-
- Dernburg, A. F., J. W. Sedat and R. S. Hawley, 1996. b Direct evidence of a role for heterochromatin in meiotic chromosome segregation. Cell 86: 135–146. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

