Genetic evidence that nonhomologous disjunction and meiotic drive are properties of wild-type Drosophila melanogaster male meiosis
- PMID: 16219792
- PMCID: PMC1456159
- DOI: 10.1534/genetics.104.036806
Genetic evidence that nonhomologous disjunction and meiotic drive are properties of wild-type Drosophila melanogaster male meiosis
Abstract
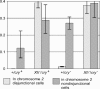
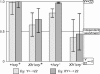
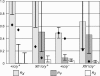
We have followed sex and second chromosome disjunction, and the effects of these chromosomes on sperm function, in four genotypes: wild-type males, males deficient for the Y-linked crystal locus, males with an X chromosome heterochromatic deficiency that deletes all X-Y pairing sites, and males with both deficiencies. Both mutant situations provoke chromosome misbehavior, but the disjunctional defects are quite different. Deficiency of the X heterochromatin, consonant with the lack of pairing sites, mostly disrupts X-Y disjunction with a decidedly second-level effect on major autosome behavior. Deleting crystal, consonant with the cytological picture of postpairing chromatin-condensation problems, disrupts sex and autosome disjunction equally. Even when the mutant-induced nondisjunction has very different mechanics, however, and even more importantly, even in the wild type, there is strong, and similar, meiotic drive. The presence of meiotic drive when disjunction is disrupted by distinctly different mechanisms supports the notion that drive is a normal cellular response to meiotic problems rather than a direct effect of particular mutants. Most surprisingly, in both wild-type and crystal-deficient males the Y chromosome moves to the opposite pole from a pair of nondisjoined second chromosomes nearly 100% of the time. This nonhomologous interaction is, however, absent when the X heterochromatin is deleted. The nonhomologous disjunction of the sex and second chromosomes may be the genetic consequence of the chromosomal compartmentalization seen by deconvolution microscopy, and the absence of Y-2 disjunction when the X heterochromatin is deleted suggests that XY pairing itself, or a previously unrecognized heterochromatic function, is prerequisite to this macrostructural organization of the chromosomes.
Figures


References
-
- Bozzetti, M. P., S. Massari, P. Finelli, F. Meggio, L. A. Pinna et al., 1995. The Ste locus, a component of the parasitic cry-Ste system of Drosophila melanogaster, encodes a protein that forms crystals in primary spermatocytes and mimics properties of the beta subunit of casein kinase 2. Proc. Natl. Acad. Sci. USA 92: 6067–6071. - PMC - PubMed
-
- Dernburg, A. F., J. W. Sedat and R. S. Hawley, 1996. b Direct evidence of a role for heterochromatin in meiotic chromosome segregation. Cell 86: 135–146. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

