Time-course of vibratory adaptation and recovery in cutaneous mechanoreceptive afferents
- PMID: 16222071
- PMCID: PMC1839047
- DOI: 10.1152/jn.00001.2005
Time-course of vibratory adaptation and recovery in cutaneous mechanoreceptive afferents
Abstract
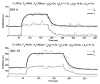
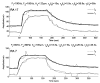
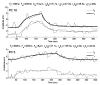
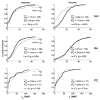
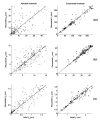
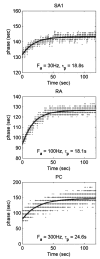
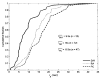
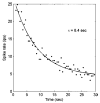
Extended suprathreshold vibratory stimulation applied to the skin results in a desensitization of cutaneous mechanoreceptive afferents. In a companion paper, we describe the dependence of the threshold shift on the parameters of the adapting stimulus and discuss neural mechanisms underlying afferent adaptation. Here we describe the time-course of afferent adaptation and recovery. We found that absolute and entrainment thresholds rise and fall exponentially during adaptation and recovery with time constants that vary with fiber type. slowly adapting type I (SA1) afferents adapt most rapidly, and pacinian (PC) afferents adapt most slowly, whereas rapidly adapting (RA) afferents exhibit intermediate rates of adaptation; SA1 fibers also recover more rapidly from adaptation than RA and PC fibers. We also showed that threshold adaptation is accompanied by a shift in the timing of the spikes within individual cycles of the adapting stimulus (i.e., a shift in the impulse phase). We invoked an integrate-and-fire model to explore possible mechanisms underlying afferent adaptation. Finally, we found that the time-course of afferent adaptation is more rapid than that of its psychophysical counterpart, as is the time-course of recovery from adaptation, suggesting that central factors play a role in the psychophysical phenomenon.
Figures

Comment in
-
Paradoxes in tactile adaptation. Focus on "vibratory adaptation in cutaneous mechanoreceptive afferents" and "time-course of vibratory adaptation and recovery in cutaneous mechanoreceptive afferents".J Neurophysiol. 2005 Nov;94(5):2995-6. doi: 10.1152/jn.00766.2005. J Neurophysiol. 2005. PMID: 16222069 No abstract available.
References
-
- Berglund U, Berglund B. Adaption and recovery in vibrotactile perception. Percept Mot Skills. 1970;30:843–853. - PubMed
-
- Bolanowski SJ, Gescheider GA, Verrillo RT, Checkosky CM. Four channels mediate the mechanical aspects of touch. J Acoust Soc Am. 1988;64:1680–1694. - PubMed
-
- Chubbuck JG. Small motion biological stimulator. Johns Hopkins APL Tech Digest. 1966;5:18–23.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

