Isolation and culture of a marine bacterium degrading the sulfated fucans from marine brown algae
- PMID: 16222488
- PMCID: PMC4273248
- DOI: 10.1007/s10126-005-5107-0
Isolation and culture of a marine bacterium degrading the sulfated fucans from marine brown algae
Abstract
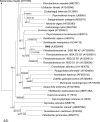
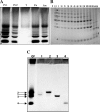
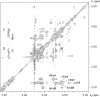
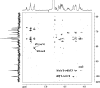
Fucoidans are matrix polysaccharides from marine brown algae, consisting of an alpha-L-fucose backbone substituted by sulfate-ester groups and masked with ramifications containing other monosaccharide residues. In spite of their interest as biologically active compounds in a number of homologous and heterologous systems, no convenient sources with fucanase activity are available yet for the degradation of the fucalean algae. We here report on the isolation, characterization, and culture conditions of a bacterial strain capable of degrading various brown algal fucoidans. This bacterium, a member of the family Flavobacteriaceae, was shown to secrete fucoidan endo-hydrolase activity. An extracellular enzyme preparation was used to degrade the fucoidan from the brown alga Pelvetia canaliculata. End products included a tetrasaccharide and a hexasaccharide made of the repetition of disaccharidic units consisting of alpha-1-->3-L-fucopyranose-2-sulfate-alpha-1-->4-L-fucopyranose-2,3-disulfate, with the 3-linked residues at the nonreducing end.
Figures


Similar articles
-
Systematic comparison of eight methods for preparation of high purity sulfated fucans extracted from the brown alga Pelvetia canaliculata.Int J Biol Macromol. 2022 Mar 15;201:143-157. doi: 10.1016/j.ijbiomac.2021.12.122. Epub 2021 Dec 27. Int J Biol Macromol. 2022. PMID: 34968546
-
In-depth structural characterization of oligosaccharides released by GH107 endofucanase MfFcnA reveals enzyme subsite specificity and sulfated fucan substructural features.Glycobiology. 2022 Mar 31;32(4):276-288. doi: 10.1093/glycob/cwab125. Glycobiology. 2022. PMID: 34939127
-
Discovery and screening of novel metagenome-derived GH107 enzymes targeting sulfated fucans from brown algae.FEBS J. 2018 Nov;285(22):4281-4295. doi: 10.1111/febs.14662. Epub 2018 Oct 8. FEBS J. 2018. PMID: 30230202
-
Structure, biology, evolution, and medical importance of sulfated fucans and galactans.Glycobiology. 2008 Dec;18(12):1016-27. doi: 10.1093/glycob/cwn085. Epub 2008 Sep 16. Glycobiology. 2008. PMID: 18796647 Review.
-
A review about brown algal cell walls and fucose-containing sulfated polysaccharides: Cell wall context, biomedical properties and key research challenges.Carbohydr Polym. 2017 Nov 1;175:395-408. doi: 10.1016/j.carbpol.2017.07.082. Epub 2017 Jul 31. Carbohydr Polym. 2017. PMID: 28917882 Review.
Cited by
-
Monoclonal antibodies directed to fucoidan preparations from brown algae.PLoS One. 2015 Feb 18;10(2):e0118366. doi: 10.1371/journal.pone.0118366. eCollection 2015. PLoS One. 2015. PMID: 25692870 Free PMC article.
-
Physicochemical and Biological Characterization of Fucoidan from Fucus vesiculosus Purified by Dye Affinity Chromatography.Mar Drugs. 2016 Apr 15;14(4):79. doi: 10.3390/md14040079. Mar Drugs. 2016. PMID: 27092514 Free PMC article.
-
Extraction of Fucoidan from Turbinaria decurrens and the Synthesis of Fucoidan-Coated AgNPs for Anticoagulant Application.ACS Omega. 2021 Nov 9;6(46):30998-31008. doi: 10.1021/acsomega.1c03776. eCollection 2021 Nov 23. ACS Omega. 2021. PMID: 34841142 Free PMC article.
-
Elevated temperature drives kelp microbiome dysbiosis, while elevated carbon dioxide induces water microbiome disruption.PLoS One. 2018 Feb 23;13(2):e0192772. doi: 10.1371/journal.pone.0192772. eCollection 2018. PLoS One. 2018. PMID: 29474389 Free PMC article.
-
Pontiella desulfatans gen. nov., sp. nov., and Pontiella sulfatireligans sp. nov., Two Marine Anaerobes of the Pontiellaceae fam. nov. Producing Sulfated Glycosaminoglycan-like Exopolymers.Microorganisms. 2020 Jun 18;8(6):920. doi: 10.3390/microorganisms8060920. Microorganisms. 2020. PMID: 32570748 Free PMC article.
References
-
- Barbeyron T, L'Haridon S, Corre E, Kloareg B, Potin P. Zobellia galactanovorans gen. nov., sp. nov., a marine species of Flavobacteriaceae isolated from a red alga, and classification of [Cytophaga] uliginosa (ZoBell and Upham, 1944) Reichenbach 1989 as Zobellia uliginosa gen. nov., comb. nov. Int J Syst Evol Microbiol. 2001;51:985–997. - PubMed
-
- Béress A, Wassermann O, Bruhn T, Béress L. A new procedure for the isolation of anti-HIV compounds (polysaccharides and polyphenols) from the marine alga Fucus vesiculosus. J Nat Prod. 1993;56:478–488. - PubMed
-
- Berteau O, McCort I, Goasdoue N, Tissot B, Daniel R. Characterization of a new alpha-l-fucosidase isolated from the marine mollusk Pecten maximus that catalyzes the hydrolysis of alpha-l-fucose from algal fucoidan (Ascophyllum nodosum) Glycobiology. 2002;12:273–282. doi: 10.1093/glycob/12.4.273. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
