Porcine acute liver failure model established by two-phase surgery and treated with hollow fiber bioartificial liver support system
- PMID: 16222738
- PMCID: PMC4320355
- DOI: 10.3748/wjg.v11.i35.5468
Porcine acute liver failure model established by two-phase surgery and treated with hollow fiber bioartificial liver support system
Abstract
Aim: To establish a highly reproducible animal model of acute liver failure (ALF), for assessing the effect of bioartificial liver support system (BALSS).
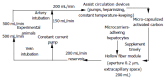
Methods: A two-phase complete liver devascularization procedure was performed in eight loco-hybrid pigs. Blood biochemical index and liver biopsy were studied every 2 h after surgery, and survival time was recorded. The BALSS constructed with high volume recirculating technique was a hollow fiber circulating system consisting of a hepatocyte reactor-hollow fiber module inoculated with microcarrier-adhering hepatocytes, and a double pump, heparinized, thermostabilized, micro-capsulized activated carbon-adsorbing plasmapheresis system. Twelve pigs undergoing two-phase surgery were randomized into: control group (perfused without hepatocytes, n = 6) and treatment group (perfused with hepatocytes, n = 6). Intergroup liver biochemical indexes, survival time, and liver pathological changes were analyzed at regular intervals.
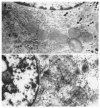
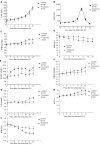
Results: Two-phase surgery was performed in all the experimental pigs, and there was no obvious difference between their biochemical indexes. After 3 h of phase II surgery, ammonia (Amm) increased to (269+/-37) micromol/L. After 5 h of the surgery, fibrinogen (Fib) decreased to (1.5+/-0.2) g/L. After 7 h of the surgery, ALT, AST, Tbil and PT were (7.6+/-1.8) nka/L, (40+/-5) nka/L, (55+/-8) micromol/L and (17.5+/-1.7) nka/L respectively. After 9 h of surgery, ALB and Cr were (27+/-4) g/L and (87+/-9) micromol/L. After 13 h of surgery, BUN was (3.5+/-0.9) micromol/L. All the above values were different from those determined before surgery. Survival time of pigs averaged 13.5+/-1.4 h. ALF pigs in the other group were treated with BALSS. The comparison analysis between the treated and control animals showed the changes of Tbil, PT, Alb, BUN, Cr, Fib, and Amm (P<0.01), but there was no change of ALT and AST. The survival time was statistically different (P<0.01), and there was no significant difference in histological changes.
Conclusion: The porcine ALF model established by two-phase devascularized surgery is valid and reproducible. The hollow fiber BALSS can meet the needs of life support and is effective in treating ALF.
Figures

Similar articles
-
Experimental research on TECA-I bioartificial liver support system to treat canines with acute liver failure.World J Gastroenterol. 2001 Oct;7(5):706-9. doi: 10.3748/wjg.v7.i5.706. World J Gastroenterol. 2001. PMID: 11819859 Free PMC article.
-
Functional evaluation of a new bioartificial liver system in vitro and in vitro.World J Gastroenterol. 2006 Feb 28;12(8):1312-6. doi: 10.3748/wjg.v12.i8.1857. World J Gastroenterol. 2006. PMID: 16534893 Free PMC article.
-
Bioartificial liver inoculated with porcine hepatocyte spheroids for treatment of canine acute liver failure model.Artif Organs. 2003 Jul;27(7):613-22. doi: 10.1046/j.1525-1594.2003.07140.x. Artif Organs. 2003. PMID: 12823416
-
Artificial and bioartificial liver support.Semin Liver Dis. 2008 May;28(2):210-7. doi: 10.1055/s-2008-1073120. Semin Liver Dis. 2008. PMID: 18452120 Review.
-
Bioartificial liver support systems for acute liver failure: A systematic review and meta-analysis of the clinical and preclinical literature.World J Gastroenterol. 2019 Jul 21;25(27):3634-3648. doi: 10.3748/wjg.v25.i27.3634. World J Gastroenterol. 2019. PMID: 31367162 Free PMC article.
Cited by
-
The multifaceted role of macrophages during acute liver injury.Front Immunol. 2023 Sep 6;14:1237042. doi: 10.3389/fimmu.2023.1237042. eCollection 2023. Front Immunol. 2023. PMID: 37736102 Free PMC article. Review.
-
An overview of animal models for investigating the pathogenesis and therapeutic strategies in acute hepatic failure.World J Gastroenterol. 2009 Jul 7;15(25):3086-98. doi: 10.3748/wjg.15.3086. World J Gastroenterol. 2009. PMID: 19575487 Free PMC article. Review.
-
Dynamic tracking of stem cells in an acute liver failure model.World J Gastroenterol. 2012 Feb 14;18(6):507-16. doi: 10.3748/wjg.v18.i6.507. World J Gastroenterol. 2012. PMID: 22363116 Free PMC article.
-
Establishment of a Novel Simplified Surgical Model of Acute Liver Failure in the Cynomolgus Monkey.Biomed Res Int. 2016;2016:3518989. doi: 10.1155/2016/3518989. Epub 2016 Dec 21. Biomed Res Int. 2016. PMID: 28097130 Free PMC article.
-
Applications of Nanobiomaterials in the Therapy and Imaging of Acute Liver Failure.Nanomicro Lett. 2020 Nov 19;13(1):25. doi: 10.1007/s40820-020-00550-x. Nanomicro Lett. 2020. PMID: 34138224 Free PMC article. Review.
References
-
- Seglen PO. Preparation of rat liver cells. 3. Enzymatic requirements for tissue dispersion. Exp Cell Res. 1973;82:391–398. - PubMed
-
- Demetriou AA, Whiting JF, Feldman D, Levenson SM, Chowdhury NR, Moscioni AD, Kram M, Chowdhury JR. Replacement of liver function in rats by transplantation of microcarrier-attached hepatocytes. Science. 1986;233:1190–1192. - PubMed
-
- Rivas-Vetencourt PA, Aranda ED, Sorio L, Quero Z, Martinez A, Vegas AM, Zerpa MJ. Xenotra-nsplantation of isolated encapsulated porcine hepatocytes in the treatment of a highly fulminant hepatic failure model. Transplant Proc. 1997;29:920–922. - PubMed
-
- Dixit V, Gitnick G. The bioartificial liver: state-of-the-art. Eur J Surg Suppl. 1998;582:71–76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

