The neurotransmitters glycine and GABA stimulate glucagon-like peptide-1 release from the GLUTag cell line
- PMID: 16223757
- PMCID: PMC1464262
- DOI: 10.1113/jphysiol.2005.098962
The neurotransmitters glycine and GABA stimulate glucagon-like peptide-1 release from the GLUTag cell line
Abstract
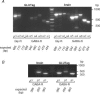
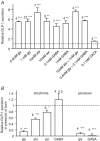
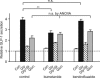
The incretin hormone, glucagon-like peptide-1 (GLP-1) is released from intestinal L-cells following food ingestion. Its secretion is triggered by a range of nutrients, including fats, carbohydrates and proteins. We reported previously that Na(+)-dependent glutamine uptake triggered electrical activity and GLP-1 release from the L-cell model line GLUTag. However, whereas alanine also triggered membrane depolarization and GLP-1 secretion, the response was Na+ independent. A range of alanine analogues, including d-alanine, beta-alanine, glycine and l-serine, but not d-serine, triggered similar depolarizing currents and elevation of intracellular [Ca2+], a sensitivity profile suggesting the involvement of glycine receptors. In support of this idea, glycine-induced currents and GLP-1 release were blocked by strychnine, and currents showed a 58.5 mV shift in reversal potential per 10-fold change in [Cl-], consistent with the activation of a Cl(-)-selective current. GABA, an agonist of related Cl- channels, also triggered Cl- currents and secretion, which were sensitive to picrotoxin. GABA-triggered [Ca2+]i increments were abolished by bicuculline and partially impaired by (1,2,5,6-tetrahydropyridine-4-yl)methylphosphinic acid (TPMPA), suggesting the involvement of both GABA(A) and GABA(C) receptors. Expression of GABA(A), GABA(C) and glycine receptor subunits was confirmed by RT-PCR. Glycine-triggered GLP-1 secretion was impaired by bumetanide but not bendrofluazide, suggesting that a high intracellular [Cl-] maintained by Na(+)-K(+)-2Cl- cotransporters is necessary for the depolarizing response to glycine receptor ligands. Our results suggest that GABA and glycine stimulate electrical activity and GLP-1 release from GLUTag cells by ligand-gated ion channel activation, a mechanism that might be important in responses to endogenous ligands from the enteric nervous system or dietary sources.
Figures




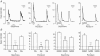

Similar articles
-
Chloride homeostasis differentially affects GABA(A) receptor- and glycine receptor-mediated effects on spontaneous circuit activity in hippocampal cell culture.Neurosci Lett. 2006 Oct 2;406(1-2):11-6. doi: 10.1016/j.neulet.2006.06.064. Epub 2006 Aug 14. Neurosci Lett. 2006. PMID: 16905250
-
Patch-clamp analysis of glycine-induced currents in chick ciliary ganglion neurons.J Physiol. 1995 Sep 1;487 ( Pt 2)(Pt 2):395-405. doi: 10.1113/jphysiol.1995.sp020888. J Physiol. 1995. PMID: 8558472 Free PMC article.
-
Effects of glycine and GABA on isolated bipolar cells of the mouse retina.J Physiol. 1990 Feb;421:645-62. doi: 10.1113/jphysiol.1990.sp017967. J Physiol. 1990. PMID: 1693403 Free PMC article.
-
The 'ABC' of GABA receptors: a brief review.Clin Exp Pharmacol Physiol. 1999 Nov;26(11):937-40. doi: 10.1046/j.1440-1681.1999.03151.x. Clin Exp Pharmacol Physiol. 1999. PMID: 10561820 Review.
-
Amino acid neurotransmitter receptor/chloride channels of mammalian sperm and the acrosome reaction.Biol Reprod. 1997 Mar;56(3):569-74. doi: 10.1095/biolreprod56.3.569. Biol Reprod. 1997. PMID: 9046998 Review.
Cited by
-
Attenuated Weight Gain with the Novel Analog of Olanzapine Linked to Sarcosinyl Moiety (PGW5) Compared to Olanzapine.J Mol Neurosci. 2016 Jan;58(1):66-73. doi: 10.1007/s12031-015-0679-2. Epub 2015 Nov 14. J Mol Neurosci. 2016. PMID: 26572534
-
Glutamine triggers and potentiates glucagon-like peptide-1 secretion by raising cytosolic Ca2+ and cAMP.Endocrinology. 2011 Feb;152(2):405-13. doi: 10.1210/en.2010-0956. Epub 2011 Jan 5. Endocrinology. 2011. PMID: 21209017 Free PMC article.
-
The Potential Role of Pancreatic γ-Aminobutyric Acid (GABA) in Diabetes Mellitus: A Critical Reappraisal.Int J Prev Med. 2021 Feb 24;12:19. doi: 10.4103/ijpvm.IJPVM_278_19. eCollection 2021. Int J Prev Med. 2021. PMID: 34084316 Free PMC article. Review.
-
Relationship between diet/exercise and pharmacotherapy to enhance the GLP-1 levels in type 2 diabetes.Endocrinol Diabetes Metab. 2019 May 16;2(3):e00068. doi: 10.1002/edm2.68. eCollection 2019 Jul. Endocrinol Diabetes Metab. 2019. PMID: 31294084 Free PMC article. Review.
-
Perspective: Prospects for Nutraceutical Support of Intestinal Barrier Function.Adv Nutr. 2021 Mar 31;12(2):316-324. doi: 10.1093/advances/nmaa139. Adv Nutr. 2021. PMID: 33126251 Free PMC article.
References
-
- Brubaker PL, Drucker DJ. Glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology. 2004;145:2653–2265. - PubMed
-
- Brubaker PL, Schloos J, Drucker DJ. Regulation of glucagon-like peptide-1 synthesis and secretion in the GLUTag enteroendocrine cell line. Endocrinology. 1998;139:4108–4114. - PubMed
-
- Chebib M. GABAC receptor ion channels. Clin Exp Pharmacol Physiol. 2004;31:800–804. - PubMed
-
- Christie GR, Ford D, Howard A, Clark MA, Hirst BH. Glycine supply to human enterocytes mediated by high-affinity basolateral GLYT1. Gastroenterology. 2001;120:439–448. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

