A comparison of release kinetics and glutamate receptor properties in shaping rod-cone differences in EPSC kinetics in the salamander retina
- PMID: 16223761
- PMCID: PMC1383429
- DOI: 10.1113/jphysiol.2005.096545
A comparison of release kinetics and glutamate receptor properties in shaping rod-cone differences in EPSC kinetics in the salamander retina
Abstract
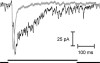
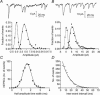
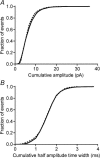
Synaptic transmission from cones is faster than transmission from rods. Using paired simultaneous recordings from photoreceptors and second-order neurones in the salamander retina, we studied the contributions of rod-cone differences in glutamate receptor properties and synaptic release rates to shaping postsynaptic responses. Depolarizing steps evoked sustained calcium currents in rods and cones that in turn produced transient excitatory postsynaptic currents (EPSCs) in horizontal and OFF bipolar cells. Cone-driven EPSCs rose and decayed faster than rod-driven EPSCs, even when comparing inputs from a rod and cone onto the same postsynaptic neurone. Thus, rod-cone differences in EPSCs reflect properties of individual rod and cone synapses. Experiments with selective AMPA and KA agonists and antagonists showed that rods and cones both contact pharmacologically similar AMPA receptors. Spontaneous miniature EPSCs (mEPSCs) exhibited unimodal distributions of amplitude and half-amplitude time width and there were no rod-cone differences in mEPSC properties. To examine how release kinetics shape the EPSC, we convolved mEPSC waveforms with empirically determined release rate functions for rods and cones. The predicted EPSC waveform closely matched the actual EPSC evoked by cones, supporting a quantal release model at the photoreceptor synapse. Convolution with the rod release function also produced a good match in rod-driven cells, although the actual EPSC was often somewhat slower than the predicted EPSC, a discrepancy partly explained by rod-rod coupling. Rod-cone differences in the rates of exocytosis are thus a major factor in producing faster cone-driven responses in second-order retinal neurones.
Figures









References
-
- Bellingham MC, Walmsley B. A novel presynaptic inhibitory mechanism underlies paired pulse depression at a fast central synapse. Neuron. 1999;23:159–170. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

