Bias from requiring explicit consent from all participants in observational research: prospective, population based study
- PMID: 16223793
- PMCID: PMC1261192
- DOI: 10.1136/bmj.38624.397569.68
Bias from requiring explicit consent from all participants in observational research: prospective, population based study
Abstract
Objective: To evaluate the differences between adults who consent to participate in observational research, and those who do not.
Design: Prospective, population based cohort study.
Setting: Primary and secondary care throughout Scotland.
Participants: 187 adults (aged > or = 16 years) resident in Scotland at the time of their first diagnosis of a brain arteriovenous malformation in 1999-2002.
Intervention: Postal consent form sent via participants' general practitioner.
Main outcome measures: Differences between consenters and non-consenters in demographic and clinical features at first presentation, and outcome during follow-up.
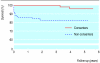
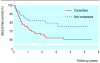
Results: 111 adults (59%) consented to participate in the study. These consenters were not significantly different from non-consenters in age, sex, or socioeconomic status at first presentation. However, consenters were significantly more likely than non-consenters to present alive and independent, and with a seizure. During follow-up, consenters were significantly more likely to receive interventional treatment. Although consenters' survival was significantly better, they were more likely to have a seizure during follow-up. Presentation with intracranial haemorrhage conferred a higher risk of subsequent haemorrhage when the whole cohort was analysed, but not when it was restricted to consenters.
Conclusions: We have found differences between adults who consent to participate in observational records-based research and those who do not, or cannot, consent. Blanket requirements for explicit consent for the use of individuals' identifiable data can bias disease registers, epidemiological studies, and health services research.
Figures
References
-
- Melton LJ III. The threat to medical-records research. N Engl J Med 1997;337: 1466-70. - PubMed
-
- Protecting patient privacy: striking a balance. Lancet 2001;358: 597. - PubMed
-
- Tu JV, Willison DJ, Silver FL, Fang J, Richards JA, Laupacis A, et al. Impracticability of informed consent in the Registry of the Canadian Stroke Network. N Engl J Med 2004;350: 1414-21. - PubMed
-
- Al-Shahi R, Bhattacharya JJ, Currie DG, Papanastassiou V, Ritchie V, Roberts RC, et al. Scottish intracranial vascular malformation study (SIVMS): evaluation of methods, ICD-10 coding, and potential sources of bias in a prospective, population-based cohort. Stroke 2003;34: 1156-62. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
