Inflammatory cells as a source of airspace extracellular superoxide dismutase after pulmonary injury
- PMID: 16224105
- PMCID: PMC2644184
- DOI: 10.1165/rcmb.2005-0212OC
Inflammatory cells as a source of airspace extracellular superoxide dismutase after pulmonary injury
Abstract
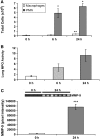
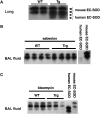
Extracellular superoxide dismutase (EC-SOD) is an antioxidant abundant in the lung. Previous studies demonstrated depletion of lung parenchymal EC-SOD in mouse models of interstitial lung disease coinciding with an accumulation of EC-SOD in airspaces. EC-SOD sticks to the matrix by a proteolytically sensitive heparin-binding domain; therefore, we hypothesized that interstitial inflammation and matrix remodeling contribute to proteolytic redistribution of EC-SOD from lung parenchyma into the airspaces. To determine if inflammation limited to airspaces leads to EC-SOD redistribution, we examined a bacterial pneumonia model. This model led to increases in airspace polymorphonuclear leukocytes staining strongly for EC-SOD. EC-SOD accumulated in airspaces at 24 h without depletion of EC-SOD from lung parenchyma. This led us to hypothesize that airspace EC-SOD was released from inflammatory cells and was not a redistribution of matrix EC-SOD. To test this hypothesis, transgenic mice with lung-specific expression of human EC-SOD were treated with asbestos or bleomycin to initiate an interstitial lung injury. In these studies, EC-SOD accumulating in airspaces was entirely the mouse isoform, demonstrating an extrapulmonary source (inflammatory cells) for this EC-SOD. We also demonstrate that EC-SOD knockout mice possess greater lung inflammation in response to bleomycin and bacteria when compared with wild types. We conclude that the source of accumulating EC-SOD in airspaces in interstitial lung disease is inflammatory cells and not the lung and that interstitial processes such as those found in pulmonary fibrosis are required to remove EC-SOD from lung matrix.
Figures






Similar articles
-
Leukocyte-derived extracellular superoxide dismutase does not contribute to airspace EC-SOD after interstitial pulmonary injury.Am J Physiol Lung Cell Mol Physiol. 2012 Jan 1;302(1):L160-6. doi: 10.1152/ajplung.00360.2010. Epub 2011 Oct 14. Am J Physiol Lung Cell Mol Physiol. 2012. PMID: 22003088 Free PMC article.
-
Redistribution of pulmonary EC-SOD after exposure to asbestos.J Appl Physiol (1985). 2004 Nov;97(5):2006-13. doi: 10.1152/japplphysiol.00480.2004. Epub 2004 Aug 6. J Appl Physiol (1985). 2004. PMID: 15298984
-
Extracellular superoxide dismutase inhibits inflammation by preventing oxidative fragmentation of hyaluronan.J Biol Chem. 2008 Mar 7;283(10):6058-66. doi: 10.1074/jbc.M709273200. Epub 2007 Dec 28. J Biol Chem. 2008. PMID: 18165226 Free PMC article.
-
Mouse extracellular superoxide dismutase: primary structure, tissue-specific gene expression, chromosomal localization, and lung in situ hybridization.Am J Respir Cell Mol Biol. 1997 Oct;17(4):393-403. doi: 10.1165/ajrcmb.17.4.2826. Am J Respir Cell Mol Biol. 1997. PMID: 9376114 Review.
-
Extracellular superoxide dismutase in pulmonary fibrosis.Antioxid Redox Signal. 2008 Feb;10(2):343-54. doi: 10.1089/ars.2007.1908. Antioxid Redox Signal. 2008. PMID: 17999630 Free PMC article. Review.
Cited by
-
Superoxide dismutase and neurological disorders.IBRO Neurosci Rep. 2024 Jan 23;16:373-394. doi: 10.1016/j.ibneur.2023.11.007. eCollection 2024 Jun. IBRO Neurosci Rep. 2024. PMID: 39007083 Free PMC article. Review.
-
Extracellular superoxide dismutase protects against matrix degradation of heparan sulfate in the lung.Antioxid Redox Signal. 2008 Feb;10(2):261-8. doi: 10.1089/ars.2007.1906. Antioxid Redox Signal. 2008. PMID: 17961072 Free PMC article.
-
Developmental expression of the receptor for advanced glycation end-products (RAGE) and its response to hyperoxia in the neonatal rat lung.BMC Dev Biol. 2007 Mar 7;7:15. doi: 10.1186/1471-213X-7-15. BMC Dev Biol. 2007. PMID: 17343756 Free PMC article.
-
Role of SOD3 in silica-related lung fibrosis and pulmonary vascular remodeling.Respir Res. 2018 Nov 20;19(1):221. doi: 10.1186/s12931-018-0933-6. Respir Res. 2018. PMID: 30453980 Free PMC article.
-
Matrix metalloproteinases promote inflammation and fibrosis in asbestos-induced lung injury in mice.Am J Respir Cell Mol Biol. 2006 Sep;35(3):289-97. doi: 10.1165/rcmb.2005-0471OC. Epub 2006 Mar 30. Am J Respir Cell Mol Biol. 2006. PMID: 16574944 Free PMC article.
References
-
- Fattman CL, Schaefer LM, Oury TD. Extracellular superoxide dismutase in biology and medicine. Free Radic Biol Med 2003;35:236–256. - PubMed
-
- Oury TD, Day BJ, Crapo JD. Extracellular superoxide dismutase in vessels and airways of humans and baboons. Free Radic Biol Med 1996;20:957–965. - PubMed
-
- Fattman CL, Enghild JJ, Crapo JD, Schaefer LM, Valnickova Z, Oury TD. Purification and characterization of extracellular superoxide dismutase in mouse lung. Biochem Biophys Res Commun 2000;275:542–548. - PubMed
-
- Fattman CL, Chang LY, Termin TA, Petersen L, Enghild JJ, Oury TD. Enhanced bleomycin-induced pulmonary damage in mice lacking extracellular superoxide dismutase. Free Radic Biol Med 2003;35:763–771. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

