Reduced dose intensity FOLFOX-4 as first line palliative chemotherapy in elderly patients with advanced colorectal cancer
- PMID: 16224154
- PMCID: PMC2779277
- DOI: 10.3346/jkms.2005.20.5.806
Reduced dose intensity FOLFOX-4 as first line palliative chemotherapy in elderly patients with advanced colorectal cancer
Abstract
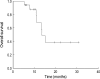
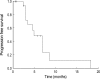
To evaluate the toxicity and efficacy of a reduced dose intensity (mini-) FOLFOX-4 regimen as a first-line palliative chemotherapy in elderly patients (> or =70 yr of age) with advanced colorectal cancer, data from prospective databases at Seoul National University Bundang Hospital and Seoul Municipal Boramae Hospital were analyzed. A total of 20 patients were enrolled between January 2001 and August 2004, and were treated with oxaliplatin 65 mg/m2 on day 1, and with 2-hr infusions of leucovorin 150 mg/m2 followed by a 5-FU bolus (300 mg/m2) and 22-hr continuous infusions (450 mg/m2) for 2 consecutive days every 2 weeks until progression, unacceptable toxicity or patient refusal. Sixteen patients were evaluable for response with an overall response rate of 43.8%. Median progression-free survival was 4.8 months (95% CI: 3.0-6.7) and overall survival was 13.5 months (95% CI: 11.1-16.0). The main side effects were anemia and neutropenia, which were observed in 20.8% and 17.7%, respectively, of the total cycles administered. There were no grade 4 toxicities and only one patient suffered from febrile neutropenia. No grade 3 toxicities occurred except for anemia (5.2%) and vomiting (1.0%). In conclusion, the mini-FOLFOX-4 regimen was found to be well tolerated with acceptable toxicity, and to provide a benefit for elderly patients with colorectal cancer.
Figures
References
-
- Jemal A, Murray T, Samuels A, Ghafoor A, Ward E, Thun MJ. Cancer statistics, 2003. CA Cancer J Clin. 2003;53:5–26. - PubMed
-
- Enzinger PC, Mayer RJ. Gastrointestinal cancer in older patients. Semin Oncol. 2004;31:206–219. - PubMed
-
- 2002 Annual Report of the Korea Central Cancer Registry. Headquarter of Korea Central Cancer Registry. [Accessed 10 May 2005]. Available from: URL: http://www.ncc.re.kr.
-
- International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. Lancet. 1995;345:939–944. - PubMed
-
- Glimelius B, Hoffman K, Graf W, Pahlman L, Sjoden PO The Nordic Gastrointestinal Tumor Adjuvant Therapy Group. Quality of life during chemotherapy in patients with symptomatic advanced colorectal cancer. Cancer. 1994;73:556–562. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical