Inability of a fusion protein of IL-2 and diphtheria toxin (Denileukin Diftitox, DAB389IL-2, ONTAK) to eliminate regulatory T lymphocytes in patients with melanoma
- PMID: 16224276
- PMCID: PMC1533764
- DOI: 10.1097/01.cji.0000175468.19742.10
Inability of a fusion protein of IL-2 and diphtheria toxin (Denileukin Diftitox, DAB389IL-2, ONTAK) to eliminate regulatory T lymphocytes in patients with melanoma
Abstract
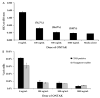
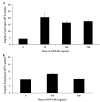
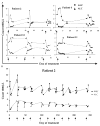
Elimination of regulatory T lymphocytes may provide a way to break self-tolerance and unleash the anti-tumor properties of circulating lymphocytes. The use of fusion proteins, which link cytotoxic molecules to receptor targets, provides one approach to this problem. This study examined the ability of a fusion protein of interleukin-2 (IL-2) and diphtheria toxin (Denileukin Diftitox, DAB389IL-2, ONTAK) to eliminate regulatory T lymphocytes based on their expression of high-affinity IL-2 receptors. Thirteen patients (12 with metastatic melanoma, 1 with metastatic renal cell carcinoma) were treated at one of the two Food and Drug Administration-approved doses of Denileukin Diftitox (seven patients at 9 microg/kg, six patients at 18 microg/kg). None of the patients experienced an objective clinical response. Foxp3 expression did not decrease significantly overall, although it did decrease minimally among patients receiving 18 microg/kg (-2.01+/-0.618 copies of Foxp3/10(3) copies of beta-actin; P=0.031). Denileukin Diftitox did not decrease the suppressive ability of CD4CD25 cells as quantified by an in vitro co-culture suppression assay. Furthermore, the increased numbers of lymphocytes in patients resulting from treatment with IL-2 were not susceptible to Denileukin Diftitox. Administration of Denileukin Diftitox does not appear to eliminate regulatory T lymphocytes or cause regression of metastatic melanoma.
Figures




References
-
- Kawaida H, Kono K, Takahashi A, et al. Distribution of CD4+CD25 high regulatory T-cells in tumor-draining lymph nodes in patients with gastric cancer. J Surg Res. 2005;124:151–157. - PubMed
-
- Jonuleit H, Schmitt E. Regulatory T-cells in antitumor therapy: Isolation and functional testing of CD4+CD25+ regulatory T-cells. Methods Mol Med. 2005;109:285–296. - PubMed
-
- Karube K, Ohshima K, Tsuchiya T, et al. Expression of Foxp3, a key molecule in CD4CD25 regulatory T cells, in adult T-cell leukaemia/lymphoma cells. Br J Haematol. 2004;126:81–84. - PubMed
-
- Wang HY, Lee DA, Peng G, et al. Tumor-specific human CD4+ regulatory T cells and their ligands: Implications for immunotherapy. Immunity. 2004;20:107–118. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

