Human skin cells support thymus-independent T cell development
- PMID: 16224538
- PMCID: PMC1253623
- DOI: 10.1172/JCI24731
Human skin cells support thymus-independent T cell development
Abstract
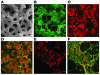
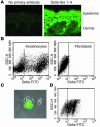
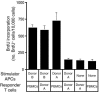
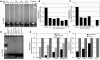
Thymic tissue has previously been considered a requirement for the generation of a functional and diverse population of human T cells. We report that fibroblasts and keratinocytes from human skin arrayed on a synthetic 3-dimensional matrix support the development of functional human T cells from hematopoietic precursor cells in the absence of thymic tissue. Newly generated T cells contained T cell receptor excision circles, possessed a diverse T cell repertoire, and were functionally mature and tolerant to self MHC, indicating successful completion of positive and negative selection. Skin cell cultures expressed the AIRE, Foxn1, and Hoxa3 transcription factors and a panel of autoantigens. Skin and bone marrow biopsies can thus be used to generate de novo functional and diverse T cell populations for potential therapeutic use in immunosuppressed patients.
Figures



Similar articles
-
Thymic microenvironments for T-cell repertoire formation.Adv Immunol. 2008;99:59-94. doi: 10.1016/S0065-2776(08)00603-2. Adv Immunol. 2008. PMID: 19117532 Review.
-
Generation of a tissue-engineered thymic organoid.Methods Mol Biol. 2007;380:163-70. doi: 10.1007/978-1-59745-395-0_9. Methods Mol Biol. 2007. PMID: 17876092 Review.
-
Positive selection in a Schnurri.Nat Immunol. 2001 Nov;2(11):989-91. doi: 10.1038/ni1101-989. Nat Immunol. 2001. PMID: 11685218 No abstract available.
-
Some quantitative aspects of T-cell repertoire selection: the requirement for regulatory T cells.Immunol Rev. 2001 Aug;182:80-8. doi: 10.1034/j.1600-065x.2001.1820106.x. Immunol Rev. 2001. PMID: 11722625 Review.
-
Central T cell tolerance: Identification of tissue-restricted autoantigens in the thymus HLA-DR peptidome.J Autoimmun. 2015 Jun;60:12-9. doi: 10.1016/j.jaut.2015.03.004. Epub 2015 Apr 21. J Autoimmun. 2015. PMID: 25911201
Cited by
-
Expression of the autoimmune regulator gene and its relevance to the mechanisms of central and peripheral tolerance.Clin Dev Immunol. 2012;2012:207403. doi: 10.1155/2012/207403. Epub 2012 Oct 22. Clin Dev Immunol. 2012. PMID: 23125865 Free PMC article. Review.
-
Prdm1 Regulates Thymic Epithelial Function To Prevent Autoimmunity.J Immunol. 2017 Aug 15;199(4):1250-1260. doi: 10.4049/jimmunol.1600941. Epub 2017 Jul 12. J Immunol. 2017. PMID: 28701508 Free PMC article.
-
Notch signalling inhibits CD4 expression during initiation and differentiation of human T cell lineage.PLoS One. 2012;7(10):e45342. doi: 10.1371/journal.pone.0045342. Epub 2012 Oct 12. PLoS One. 2012. PMID: 23071513 Free PMC article.
-
T cells fail to develop in the human skin-cell explants system; an inconvenient truth.BMC Immunol. 2011 Feb 18;12:17. doi: 10.1186/1471-2172-12-17. BMC Immunol. 2011. PMID: 21332988 Free PMC article.
-
A simple model system enabling human CD34(+) cells to undertake differentiation towards T cells.PLoS One. 2013 Jul 23;8(7):e69572. doi: 10.1371/journal.pone.0069572. Print 2013. PLoS One. 2013. PMID: 23894504 Free PMC article.
References
-
- Heitger A, et al. Essential role of the thymus to reconstitute naive (CD45RA+) T-helper cells after human allogeneic bone marrow transplantation. Blood. 1997;90:850–857. - PubMed
-
- von Gaudecker B. Ultrastructure of the age-involuted adult human thymus. Cell Tissue Res. 1978;186:507–525. - PubMed
-
- Galy A, Travis M, Cen D, Chen B. Human T, B, natural killer, and dendritic cells arise from a common bone marrow progenitor cell subset. Immunity. 1995;3:459–473. - PubMed
-
- Kingston R, Jenkinson EJ, Owen JJ. A single stem cell can recolonize an embryonic thymus, producing phenotypically distinct T-cell populations. Nature. 1985;317:811–813. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

