Combretastatin A4 phosphate induces rapid regression of tumor neovessels and growth through interference with vascular endothelial-cadherin signaling
- PMID: 16224539
- PMCID: PMC1253622
- DOI: 10.1172/JCI24586
Combretastatin A4 phosphate induces rapid regression of tumor neovessels and growth through interference with vascular endothelial-cadherin signaling
Abstract
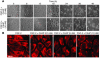
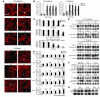
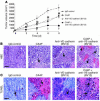
The molecular and cellular pathways that support the maintenance and stability of tumor neovessels are not well defined. The efficacy of microtubule-disrupting agents, such as combretastatin A4 phosphate (CA4P), in inducing rapid regression of specific subsets of tumor neovessels has opened up new avenues of research to identify factors that support tumor neoangiogenesis. Herein, we show that CA4P selectively targeted endothelial cells, but not smooth muscle cells, and induced regression of unstable nascent tumor neovessels by rapidly disrupting the molecular engagement of the endothelial cell-specific junctional molecule vascular endothelial-cadherin (VE-cadherin) in vitro and in vivo in mice. CA4P increases endothelial cell permeability, while inhibiting endothelial cell migration and capillary tube formation predominantly through disruption of VE-cadherin/beta-catenin/Akt signaling pathway, thereby leading to rapid vascular collapse and tumor necrosis. Remarkably, stabilization of VE-cadherin signaling in endothelial cells with adenovirus E4 gene or ensheathment with smooth muscle cells confers resistance to CA4P. CA4P synergizes with low and nontoxic doses of neutralizing mAbs to VE-cadherin by blocking assembly of neovessels, thereby inhibiting tumor growth. These data suggest that the microtubule-targeting agent CA4P selectively induces regression of unstable tumor neovessels, in part through disruption of VE-cadherin signaling. Combined treatment with anti-VE-cadherin agents in conjunction with microtubule-disrupting agents provides a novel synergistic strategy to selectively disrupt assembly and induce regression of nascent tumor neovessels, with minimal toxicity and without affecting normal stabilized vasculature.
Figures






Similar articles
-
Adrenomedullin blockade induces regression of tumor neovessels through interference with vascular endothelial-cadherin signalling.Oncotarget. 2015 Apr 10;6(10):7536-53. doi: 10.18632/oncotarget.3167. Oncotarget. 2015. PMID: 25924235 Free PMC article.
-
Vascular disrupting effect of combretastatin A-4 phosphate with inhibition of vascular endothelial cadherin in canine osteosarcoma-xenografted mice.Res Vet Sci. 2019 Feb;122:1-6. doi: 10.1016/j.rvsc.2018.10.017. Epub 2018 Oct 30. Res Vet Sci. 2019. PMID: 30439557
-
Selective targeting of angiogenic tumor vasculature by vascular endothelial-cadherin antibody inhibits tumor growth without affecting vascular permeability.Cancer Res. 2002 May 1;62(9):2567-75. Cancer Res. 2002. PMID: 11980651
-
Combretastatin A4 phosphate: a novel vascular disrupting agent.Future Oncol. 2010 Aug;6(8):1219-28. doi: 10.2217/fon.10.90. Future Oncol. 2010. PMID: 20799867 Review.
-
Combretastatin A4 phosphate.Anticancer Drugs. 2004 Mar;15(3):179-87. doi: 10.1097/00001813-200403000-00001. Anticancer Drugs. 2004. PMID: 15014350 Review.
Cited by
-
Combretastatin A-4 inhibits cell growth and metastasis in bladder cancer cells and retards tumour growth in a murine orthotopic bladder tumour model.Br J Pharmacol. 2010 Aug;160(8):2008-27. doi: 10.1111/j.1476-5381.2010.00861.x. Br J Pharmacol. 2010. PMID: 20649598 Free PMC article.
-
Synthesis of structurally diverse benzosuberene analogues and their biological evaluation as anti-cancer agents.Bioorg Med Chem. 2013 Dec 15;21(24):8019-32. doi: 10.1016/j.bmc.2013.08.035. Epub 2013 Sep 4. Bioorg Med Chem. 2013. PMID: 24183586 Free PMC article.
-
Fasentin diminishes endothelial cell proliferation, differentiation and invasion in a glucose metabolism-independent manner.Sci Rep. 2020 Apr 9;10(1):6132. doi: 10.1038/s41598-020-63232-z. Sci Rep. 2020. PMID: 32273578 Free PMC article.
-
Vascular disrupting agent drug classes differ in effects on the cytoskeleton.PLoS One. 2012;7(7):e40177. doi: 10.1371/journal.pone.0040177. Epub 2012 Jul 24. PLoS One. 2012. PMID: 22848372 Free PMC article.
-
Structure-activity relationship and in vitro and in vivo evaluation of the potent cytotoxic anti-microtubule agent N-(4-methoxyphenyl)-N,2,6-trimethyl-6,7-dihydro-5H-cyclopenta[d]pyrimidin-4-aminium chloride and its analogues as antitumor agents.J Med Chem. 2013 Sep 12;56(17):6829-44. doi: 10.1021/jm400639z. Epub 2013 Aug 29. J Med Chem. 2013. PMID: 23895532 Free PMC article.
References
-
- Zhu Z, et al. Inhibition of human leukemia in an animal model with human antibodies directed against vascular endothelial growth factor receptor 2. Correlation between antibody affinity and biological activity. Leukemia. 2003;17:604–611. - PubMed
-
- Breviario F, et al. Functional properties of human vascular endothelial cadherin (7B4/cadherin-5), an endothelium-specific cadherin. Arterioscler. Thromb. Vasc. Biol. 1995;15:1229–1239. - PubMed
-
- Carmeliet P, et al. Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell. 1999;98:147–157. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

