Rescue of cell growth by sphingosine with disruption of lipid microdomain formation in Saccharomyces cerevisiae deficient in sphingolipid biosynthesis
- PMID: 16225461
- PMCID: PMC1386021
- DOI: 10.1042/BJ20051354
Rescue of cell growth by sphingosine with disruption of lipid microdomain formation in Saccharomyces cerevisiae deficient in sphingolipid biosynthesis
Abstract
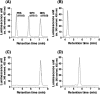
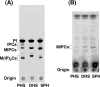
In the yeast Saccharomyces cerevisiae, sphingolipids are essential for cell growth. Inactivation of sphingolipid biosynthesis, such as by disrupting the serine palmitoyltransferase gene (LCB2), is lethal, but cells can be rescued by supplying an exogenous LCB (long-chain base) like PHS (phytosphingosine) or DHS (dihydrosphingosine). In the present study, supplying SPH (sphingosine), an unnatural LCB for yeast, similarly rescued the Deltalcb2 cells, but only when SPH 1-phosphate production was inhibited by deleting the LCB kinase gene LCB4. Exogenously added SPH was adequately converted into phosphoinositol-containing complex sphingolipids. Interestingly, cells carrying SPH-based sphingolipids exhibited a defect in the association of Pma1p with Triton X-100-insoluble membrane fractions, and displayed sensitivities to both Ca2+ and hygromycin B. These results suggest that the SPH-based sphingolipids in these cells have properties that differ from those of the PHS- or DHS-based sphingolipids in regard to lipid microdomain formation, leading to abnormal sensitivities towards certain environmental stresses. The present paper is the first report showing that in sphingolipid-deficient S. cerevisiae, the requirement for LCB can be fulfilled by exogenous SPH, although this supplement results in failure of lipid microdomain formation.
Figures



References
-
- Degroote S., Wolthoorn J., van Meer G. The cell biology of glycosphingolipids. Semin. Cell Dev. Biol. 2004;15:375–387. - PubMed
-
- Simons K., Ikonen E. Functional rafts in cell membranes. Nature (London) 1997;387:569–572. - PubMed
-
- Merrill A. H., Jr De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J. Biol. Chem. 2002;277:25843–25846. - PubMed
-
- Dickson R. C., Lester R. L. Yeast sphingolipids. Biochim. Biophys. Acta. 1999;1426:347–357. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

