Contribution of trafficking signals in the cytoplasmic tail of the infectious bronchitis virus spike protein to virus infection
- PMID: 16227244
- PMCID: PMC1262608
- DOI: 10.1128/JVI.79.21.13209-13217.2005
Contribution of trafficking signals in the cytoplasmic tail of the infectious bronchitis virus spike protein to virus infection
Abstract
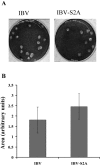
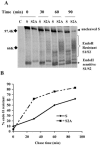
Coronavirus spike (S) proteins are responsible for binding and fusion with target cells and thus play an essential role in virus infection. Recently, we identified a dilysine endoplasmic reticulum (ER) retrieval signal and a tyrosine-based endocytosis signal in the cytoplasmic tail of the S protein of infectious bronchitis virus (IBV). Here, an infectious cDNA clone of IBV was used to address the importance of the S protein trafficking signals to virus infection. We constructed infectious cDNA clones lacking the ER retrieval signal, the endocytosis signal, or both. The virus lacking the ER retrieval signal was viable. However, this virus had a growth defect at late times postinfection and produced larger plaques than IBV. Further analysis confirmed that the mutant S protein trafficked though the secretory pathway faster than wild-type S protein. A more dramatic phenotype was obtained when the endocytosis signal was mutated. Recombinant viruses lacking the endocytosis signal (in combination with a mutated dilysine signal or alone) could not be recovered, even though transient syncytia were formed in transfected cells. Our results suggest that the endocytosis signal of IBV S is essential for productive virus infection.
Figures







References
-
- Bonifacino, J. S., and L. M. Traub. 2003. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72:395-447. - PubMed
-
- Danis, C., J. Deschambeault, S. Do Carmo, E. A. Cohen, E. Rassart, and G. Lemay. 2004. The tyrosine-based YXXO targeting motif of murine leukemia virus envelope glycoprotein affects pathogenesis. Virology 324:173-183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

