VP24 of Marburg virus influences formation of infectious particles
- PMID: 16227263
- PMCID: PMC1262563
- DOI: 10.1128/JVI.79.21.13421-13433.2005
VP24 of Marburg virus influences formation of infectious particles
Abstract
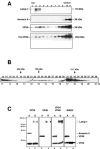
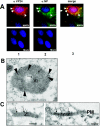
The highly pathogenic enveloped Marburg virus (MARV) is composed of seven structural proteins and the nonsegmented negative-sense viral RNA genome. Four proteins (NP, VP35, VP30, and L) make up the helical nucleocapsid, which is surrounded by a matrix that is composed of the viral proteins VP40 and VP24. VP40 is functionally homologous to the matrix proteins of other nonsegmented negative-strand RNA viruses. As yet, the function of VP24 remains elusive. In the present study we found that VP24 colocalized with inclusions in MARV-infected cells that contain preformed nucleocapsids and with nucleocapsids outside the inclusions. Coexpression studies revealed that VP24 is recruited into the inclusions by the presence of NP. Furthermore, VP24 displayed membrane-binding properties and was recruited into filamentous virus-like particles (VLPs) that are induced by VP40. The incorporation of VP24 altered neither the morphology of VLPs nor the budding efficiency of VLPs. When VP24 was silenced in MARV-infected cells by small interfering RNA technology, the release of viral particles was significantly reduced while viral transcription and replication were unimpaired. Our data support the idea that VP24 is essential for a process that takes place after replication and transcription and before budding of virus progeny. It is presumed that VP24 is necessary for the formation of transport-competent nucleocapsids and/or the interaction between the nucleocapsids and the budding sites at the plasma membrane.
Figures

References
-
- Becker, S., C. Rinne, U. Hofsass, H. D. Klenk, and E. Mühlberger. 1998. Interactions of Marburg virus nucleocapsid proteins. Virology 249:406-417. - PubMed
-
- Bordier, C. 1981. Phase separation of integral membrane proteins in Triton X-114 solution. J. Biol. Chem. 256:1604-1607. - PubMed
-
- Cheusova, T. B., S. Becker, E. Muehlberger, and E. I. Ryabchikova. 2002. Submicroscopic characteristics of Marburg virus and its mini genome analog replication in cell cultures. Mol. Gen. Mikrobiol. Virusol. 2:27-30. (In Russian.) - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

