Identification, subviral localization, and functional characterization of the pseudorabies virus UL17 protein
- PMID: 16227265
- PMCID: PMC1262560
- DOI: 10.1128/JVI.79.21.13442-13453.2005
Identification, subviral localization, and functional characterization of the pseudorabies virus UL17 protein
Abstract
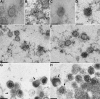
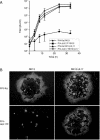
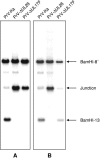
Homologs of the UL17 gene of the alphaherpesvirus herpes simplex virus 1 (HSV-1) are conserved in all three subfamilies of herpesviruses. However, only the HSV-1 protein has so far been characterized in any detail. To analyze UL17 of pseudorabies virus (PrV) the complete 597-amino-acid protein was expressed in Escherichia coli and used for rabbit immunization. The antiserum recognized a 64-kDa protein in PrV-infected cell lysates and purified virions, identifying PrV UL17 as a structural virion component. In indirect immunofluorescence analyses of PrV-infected cells the protein was predominantly found in the nucleus. In electron microscopic studies after immunogold labeling of negatively stained purified virion preparations, UL17-specific label was detected on single, mostly damaged capsids, whereas complete virions and the majority of capsids were free of label. In ultrathin sections of infected cells, label was primarily found dispersed around scaffold-containing B-capsids, whereas on DNA-filled C-capsids it was located in the center. Empty intranuclear A-capsids were free of label, as were extracellular capsid-less L-particles. Functional characterization of PrV-DeltaUL17F, a deletion mutant lacking codons 23 to 444, demonstrated that cleavage of viral DNA into unit-length genomes was inhibited in the absence of UL17. In electron microscopic analyses of PrV-DeltaUL17F-infected RK13 cells, DNA-containing capsids were not detected, while numerous capsidless L-particles were observed. In summary, our data indicate that the PrV UL17 protein is an internal nucleocapsid protein necessary for DNA cleavage and packaging but suggest that the protein is not a prominent part of the tegument.
Figures





References
-
- Al-Kobaisi, M. F., F. J. Rixon, I. McDougall, and V. G. Preston. 1991. The herpes simplex virus UL33 gene product is required for the assembly of full capsids. Virology 180:380-388. - PubMed
-
- Baer, R., A. T. Bankier, M. D. Biggin, P. L. Deininger, P. J. Farrell, T. J. Gibson, G. F. Hatfull, G. S. Hudson, S. C. Satchwell, C. Seguin, P. Tuffnell, and B. G. Barrell. 1984. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature (London) 310:207-211. - PubMed
-
- Baines, J. D., and S. K. Weller. 2005. Cleavage and packaging of herpes simplex virus 1 DNA. In C. E. Catalano (ed.), Viral genome packaging machines: genetics, structures, and mechanism. Landes Biosciences, Georgetown, Tex.
-
- Beard, P. M., and J. D. Baines. 2004. The DNA cleavage and packaging protein encoded by the UL33 gene of herpes simplex virus 1 associates with capsids. Virology 324:475-482. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

