Activation of Kaposi's sarcoma-associated herpesvirus lytic gene expression during epithelial differentiation
- PMID: 16227296
- PMCID: PMC1262565
- DOI: 10.1128/JVI.79.21.13769-13777.2005
Activation of Kaposi's sarcoma-associated herpesvirus lytic gene expression during epithelial differentiation
Abstract
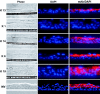
The oral cavity has been identified as the major site for the shedding of infectious Kaposi's sarcoma-associated herpesvirus (KSHV). While KSHV DNA is frequently detected in the saliva of KSHV seropositive persons, it does not appear to replicate in salivary glands. Some viruses employ the process of epithelial differentiation for productive viral replication. To test if KSHV utilizes the differentiation of oral epithelium as a mechanism for the activation of lytic replication and virus production, we developed an organotypic raft culture model of epithelium using keratinocytes from human tonsils. This system produced a nonkeratinized stratified squamous oral epithelium in vitro, as demonstrated by the presence of nucleated cells at the apical surface; the expression of involucrin and keratins 6, 13, 14, and 19; and the absence of keratin 1. The activation of KSHV lytic-gene expression was examined in this system using rKSHV.219, a recombinant virus that expresses the green fluorescent protein during latency from the cellular EF-1alpha promoter and the red fluorescent protein (RFP) during lytic replication from the viral early PAN promoter. Infection of keratinocytes with rKSHV.219 resulted in latent infection; however, when these keratinocytes differentiated into a multilayered epithelium, lytic cycle activation of rKSHV.219 occurred, as evidenced by RFP expression, the expression of the late virion protein open reading frame K8.1, and the production of infectious rKSHV.219 at the epithelial surface. These findings demonstrate that KSHV lytic activation occurs as keratinocytes differentiate into a mature epithelium, and it may be responsible for the presence of infectious KSHV in saliva.
Figures






References
-
- Andreoni, M., G. El-Sawaf, G. Rezza, B. Ensoli, E. Nicastri, L. Ventura, L. Ercoli, L. Sarmati, and G. Rocchi. 1999. High seroprevalence of antibodies to human herpesvirus-8 in Egyptian children: evidence of nonsexual transmission. J. Natl. Cancer Inst. 91:465-469. - PubMed
-
- Andreoni, M., L. Sarmati, E. Nicastri, G. El Sawaf, M. El Zalabani, I. Uccella, R. Bugarini, S. G. Parisi, and G. Rezza. 2002. Primary human herpesvirus 8 infection in immunocompetent children. JAMA 287:1295-1300. - PubMed
-
- Asselineau, D., and M. Prunieras. 1984. Reconstruction of ‘simplified’ skin: control of fabrication. Br. J. Dermatol. 111(Suppl. 27):219-222. - PubMed
-
- Bell, E., S. Sher, B. Hull, C. Merrill, S. Rosen, A. Chamson, D. Asselineau, L. Dubertret, B. Coulomb, C. Lapiere, B. Nusgens, and Y. Neveux. 1983. The reconstitution of living skin. J. Investig. Dermatol. 81:2S-10S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

