Clinical efficacy of anti-pneumococcal vaccination in patients with COPD
- PMID: 16227328
- PMCID: PMC2080738
- DOI: 10.1136/thx.2005.043323
Clinical efficacy of anti-pneumococcal vaccination in patients with COPD
Abstract
Background: A study was undertaken to evaluate the clinical efficacy of the 23-valent pneumococcal polysaccharide vaccine (PPV) in immunocompetent patients with chronic obstructive pulmonary disease (COPD).
Methods: A randomised controlled trial was carried out in 596 patients with COPD of mean (SD) age 65.8 (9.7) years, 298 of whom received PPV. The main outcome was radiographically proven community acquired pneumonia (CAP) of pneumococcal or unknown aetiology after a mean period of 979 days (range 20-1454).
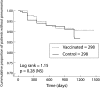
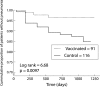
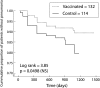
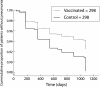
Results: There were 58 first episodes of CAP caused by pneumococcus or of unknown aetiology, 25 in the intervention group and 33 in the non-intervention group. Kaplan-Meier survival curves for CAP did not show significant differences between the intervention and non-intervention arms (log rank test = 1.15, p = 0.28) in the whole group of patients. The efficacy of PPV in all patients was 24% (95% CI -24 to 54; p = 0.333). In the subgroup aged <65 years the efficacy of PPV was 76% (95% CI 20 to 93; p = 0.013), while in those with severe functional obstruction (forced expiratory volume in 1 second <40%) it was 48% (95% CI -7 to 80; p = 0.076). In younger patients with severe airflow obstruction the efficacy was 91% (95% CI 35 to 99; p = 0.002). There were only five cases of non-bacteraemic pneumococcal CAP, all in the non-intervention group (log rank test = 5.03; p = 0.025). Multivariate analysis gave a hazard ratio for unknown and pneumococcal CAP in the vaccinated group, adjusted for age, of 0.20 (95% CI 0.06 to 0.68; p = 0.01).
Conclusions: PPV is effective in preventing CAP in patients with COPD aged less than 65 years and in those with severe airflow obstruction. No differences were found among the other groups of patients with COPD.
Conflict of interest statement
Competing interests: none declared.
Comment in
-
Polysaccharide pneumococcal vaccination: new evidence.Thorax. 2006 Mar;61(3):183-4. doi: 10.1136/thx.2005.046318. Thorax. 2006. PMID: 16517581 Free PMC article.
References
-
- Anon Pneumococcal vaccines. WHO position paper. Wkly Epidemiol Rec 199974177–183. - PubMed
-
- Austrian R, Gold J. Pneumococcal bacteremia with special reference to bacteremic pneumococcal pneumonia. Ann Intern Med 196460759–776. - PubMed
-
- Ortqvist A. Pneumococcal vaccination: current and future issues. Eur Respir J 200118184–195. - PubMed
-
- Lipsky B A, Boyko E J, Inui T S.et al Risk factors for acquiring pneumococcal infections. Arch Intern Med 19861462179–2185. - PubMed
-
- Pastor P, Medley F, Murphy T V. Invasive pneumococcal disease in Dallas County, Texas: results from population‐based surveillance in 1995. Clin Infect Dis 199826590–595. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous