Micro-RNA regulation of the mammalian lin-28 gene during neuronal differentiation of embryonal carcinoma cells
- PMID: 16227573
- PMCID: PMC1265813
- DOI: 10.1128/MCB.25.21.9198-9208.2005
Micro-RNA regulation of the mammalian lin-28 gene during neuronal differentiation of embryonal carcinoma cells
Abstract
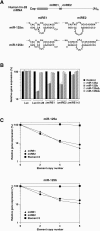
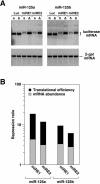
Vertebrate genomes each encode hundreds of micro-RNAs (miRNAs), yet for few of these miRNAs is there empirical evidence as to which mRNA(s) they regulate. Here we report the identification of human lin-28 mRNA as a regulatory target of human miR-125b and its homolog miR-125a. Studies of miR-125b function in mouse P19 embryonal carcinoma cells induced to develop into neurons suggest a role for this regulatory miRNA in mammalian neuronal differentiation, since its increased concentration in these cells contributes to lin-28 downregulation. Within the lin-28 3' untranslated region (UTR) are two conserved miRNA responsive elements (miREs) that mediate repression by miR-125b and miR-125a. Simultaneous deletion of both miREs renders the lin-28 3' UTR almost completely insensitive to these miRNAs, indicating that these two miREs are the principal elements in the lin-28 3' UTR that respond to miR-125. At the 3' end of each element is an adenosine residue that makes a significant contribution to function irrespective of its complementarity to the 5'-terminal nucleotide of miR-125. By contrast to most earlier reports of gene repression by other miRNAs that are imperfectly complementary to their targets, lin-28 downregulation by miR-125 involves reductions in both translational efficiency and mRNA abundance. The decrease in the mRNA concentration is achieved by a posttranscriptional mechanism that is independent of the inhibitory effect on translation.
Figures









References
-
- Ambros, V., and H. R. Horvitz. 1984. Heterochronic mutants of the nematode Caenorhabditis elegans. Science 226:409-416. - PubMed
-
- Berezikov, E., V. Guryev, J. van de Belt, E. Wienholds, R. H. Plasterk, and E. Cuppen. 2005. Phylogenetic shadowing and computational identification of human microRNA genes. Cell 120:21-24. - PubMed
-
- Brewer, G. J., J. R. Torricelli, E. K. Evege, and P. J. Price. 1993. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J. Neurosci. Res. 35:567-576. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
