Clathrin adaptor AP-2 is essential for early embryonal development
- PMID: 16227583
- PMCID: PMC1265839
- DOI: 10.1128/MCB.25.21.9318-9323.2005
Clathrin adaptor AP-2 is essential for early embryonal development
Abstract
The heterotetrameric adaptor protein (AP) complexes AP-1, AP-2, AP-3, and AP-4 play key roles in transport vesicle formation and cargo sorting in post-Golgi trafficking pathways. Studies on cultured mammalian cells have shown that AP-2 mediates rapid endocytosis of a subset of plasma membrane receptors. To determine whether this function is essential in the context of a whole mammalian organism, we carried out targeted disruption of the gene encoding the mu2 subunit of AP-2 in the mouse. We found that mu2 heterozygous mutant mice were viable and had an apparently normal phenotype. In contrast, no mu2 homozygous mutant embryos were identified among blastocysts from intercrossed heterozygotes, indicating that mu2-deficient embryos die before day 3.5 postcoitus (E3.5). These results indicate that AP-2 is indispensable for early embryonic development, which might be due to its requirement for cell viability.
Figures
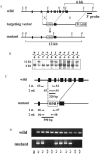
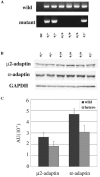
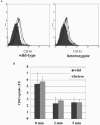
References
-
- Adra, C. N., P. H. Boer, and M. W. McBurney. 1987. Cloning and expression of the mouse pgk-1 gene and the nucleotide sequence of its promoter. Gene 60:65-74. - PubMed
-
- Aguilar, R. C., H. Ohno, K. W. Roche, and J. S. Bonifacino. 1997. Functional domain mapping of the clathrin-associated adaptor medium chains μ1 and μ2. J. Biol. Chem. 272:27160-27166. - PubMed
-
- Brodsky, F. M., C. Y. Chen, C. Knuehl, M. C. Towler, and D. E. Wakeham. 2001. Biological basket weaving: formation and function of clathrin-coated vesicles. Annu. Rev. Cell Dev. Biol. 17:517-568. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
