Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands
- PMID: 16227625
- PMCID: PMC2266087
- DOI: 10.1074/jbc.M504212200
Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands
Abstract
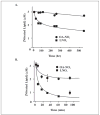
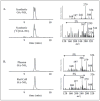
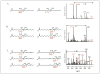
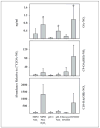
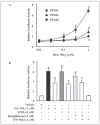
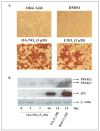
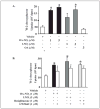
Mass spectrometric analysis of human plasma and urine revealed abundant nitrated derivatives of all principal unsaturated fatty acids. Nitrated palmitoleic, oleic, linoleic, linolenic, arachidonic and eicosapentaenoic acids were detected in concert with their nitrohydroxy derivatives. Two nitroalkene derivatives of the most prevalent fatty acid, oleic acid, were synthesized (9- and 10-nitro-9-cis-octadecenoic acid; OA-NO2), structurally characterized and determined to be identical to OA-NO2 found in plasma, red cells, and urine of healthy humans. These regioisomers of OA-NO2 were quantified in clinical samples using 13C isotope dilution. Plasma free and esterified OA-NO2 concentrations were 619 +/- 52 and 302 +/- 369 nm, respectively, and packed red blood cell free and esterified OA-NO2 was 59 +/- 11 and 155 +/- 65 nm. The OA-NO2 concentration of blood is approximately 50% greater than that of nitrated linoleic acid, with the combined free and esterified blood levels of these two fatty acid derivatives exceeding 1 microm. OA-NO2 is a potent ligand for peroxisome proliferator activated receptors at physiological concentrations. CV-1 cells co-transfected with the luciferase gene under peroxisome proliferator-activated receptor (PPAR) response element regulation, in concert with PPARgamma, PPARalpha, or PPARdelta expression plasmids, showed dose-dependent activation of all PPARs by OA-NO2. PPARgamma showed the greatest response, with significant activation at 100 nm, while PPARalpha and PPARdelta were activated at approximately 300 nm OA-NO2. OA-NO2 also induced PPAR gamma-dependent adipogenesis and deoxyglucose uptake in 3T3-L1 preadipocytes at a potency exceeding nitrolinoleic acid and rivaling synthetic thiazo-lidinediones. These data reveal that nitrated fatty acids comprise a class of nitric oxide-derived, receptor-dependent, cell signaling mediators that act within physiological concentration ranges.
Figures




References
-
- Smith WL. Am J Physiol. 1992;263:F181–F191. - PubMed
-
- Montuschi P, Barnes PJ, Roberts LJ. FASEB J. 2004;18:1791–1800. - PubMed
-
- Rubbo H, Radi R, Trujillo M, Telleri R, Kalyanaraman B, Barnes S, Kirk M, Freeman BA. J Biol Chem. 1994;269:26066–26075. - PubMed
-
- Schopfer FJ, Baker PR, Freeman BA. Trends Biochem Sci. 2003;28:646–654. - PubMed
-
- Marshall HE, Merchant K, Stamler JS. FASEB J. 2000;14:1889–1900. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL03676/HL/NHLBI NIH HHS/United States
- T32HL07457/HL/NHLBI NIH HHS/United States
- R25 HL003676/HL/NHLBI NIH HHS/United States
- S06 GM008248/GM/NIGMS NIH HHS/United States
- HL64937/HL/NHLBI NIH HHS/United States
- HL075397/HL/NHLBI NIH HHS/United States
- S06GM08248/GM/NIGMS NIH HHS/United States
- R01 HL058115/HL/NHLBI NIH HHS/United States
- R01 HL068878/HL/NHLBI NIH HHS/United States
- R37 HL058115/HL/NHLBI NIH HHS/United States
- UH1 HL003676/HL/NHLBI NIH HHS/United States
- R01 HL064937/HL/NHLBI NIH HHS/United States
- R01 HL075397/HL/NHLBI NIH HHS/United States
- HL58115/HL/NHLBI NIH HHS/United States
- HL068878/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

