Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo
- PMID: 16227992
- PMCID: PMC2277080
- DOI: 10.1038/nm1307
Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo
Abstract
Akt kinases control essential cellular functions, including proliferation, apoptosis, metabolism and transcription, and have been proposed as promising targets for treatment of angiogenesis-dependent pathologies, such as cancer and ischemic injury. But their precise roles in neovascularization remain elusive. Here we show that Akt1 is the predominant isoform in vascular cells and describe the unexpected consequences of Akt1 knockout on vascular integrity and pathological angiogenesis. Angiogenic responses in three distinct in vivo models were enhanced in Akt1(-/-) mice; these enhanced responses were associated with impairment of blood vessel maturation and increased vascular permeability. Although impaired vascular maturation in Akt1(-/-) mice may be attributed to reduced activation of endothelial nitric oxide synthase (eNOS), the major phenotypic changes in vascular permeability and angiogenesis were linked to reduced expression of two endogenous vascular regulators, thrombospondins 1 (TSP-1) and 2 (TSP-2). Re-expression of TSP-1 and TSP-2 in mice transplanted with wild-type bone marrow corrected the angiogenic abnormalities in Akt1(-/-) mice. These findings establish a crucial role of an Akt-thrombospondin axis in angiogenesis.
Figures




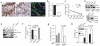

References
-
- Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. - PubMed
-
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995;1:27–31. - PubMed
-
- Carmeliet P. Angiogenesis in health and disease. Nat. Med. 2003;9:653–660. - PubMed
-
- Kandel ES, Hay N. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp. Cell Res. 1999;253:210–229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

