Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis
- PMID: 16230474
- PMCID: PMC2213215
- DOI: 10.1084/jem.20050977
Innate immunity against Francisella tularensis is dependent on the ASC/caspase-1 axis
Abstract
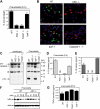
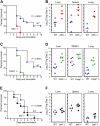
Francisella tularensis is a highly infectious gram-negative coccobacillus that causes the zoonosis tularemia. This bacterial pathogen causes a plague-like disease in humans after exposure to as few as 10 cells. Many of the mechanisms by which the innate immune system fights Francisella are unknown. Here we show that wild-type Francisella, which reach the cytosol, but not Francisella mutants that remain localized to the vacuole, induced a host defense response in macrophages, which is dependent on caspase-1 and the death-fold containing adaptor protein ASC. Caspase-1 and ASC signaling resulted in host cell death and the release of the proinflammatory cytokines interleukin (IL)-1beta and IL-18. F. tularensis-infected caspase-1- and ASC-deficient mice showed markedly increased bacterial burdens and mortality as compared with wild-type mice, demonstrating a key role for caspase-1 and ASC in innate defense against infection by this pathogen.
Figures




References
-
- Sjostedt, A. 2003. Virulence determinants and protective antigens of Francisella tularensis. Curr. Opin. Microbiol. 6:66–71. - PubMed
-
- Elkins, K.L., S.C. Cowley, and C.M. Bosio. 2003. Innate and adaptive immune responses to an intracellular bacterium, Francisella tularensis live vaccine strain. Microbes Infect. 5:135–142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

