Chemokine receptor CCR5 promotes leukocyte trafficking to the brain and survival in West Nile virus infection
- PMID: 16230476
- PMCID: PMC2213214
- DOI: 10.1084/jem.20042530
Chemokine receptor CCR5 promotes leukocyte trafficking to the brain and survival in West Nile virus infection
Abstract
The molecular immunopathogenesis of West Nile virus (WNV) infection is poorly understood. Here, we characterize a mouse model for WNV using a subcutaneous route of infection and delineate leukocyte subsets and immunoregulatory factors present in the brains of infected mice. Central nervous system (CNS) expression of the chemokine receptor CCR5 and its ligand CCL5 was prominently up-regulated by WNV, and this was associated with CNS infiltration of CD4+ and CD8+ T cells, NK1.1+ cells and macrophages expressing the receptor. The significance of CCR5 in pathogenesis was established by mortality studies in which infection of CCR5-/- mice was rapidly and uniformly fatal. In the brain, WNV-infected CCR5-/- mice had increased viral burden but markedly reduced NK1.1+ cells, macrophages, and CD4+ and CD8+ T cells compared with WNV-infected CCR5+/+ mice. Adoptive transfer of splenocytes from WNV-infected CCR5+/+ mice into infected CCR5-/- mice increased leukocyte accumulation in the CNS compared with transfer of splenocytes from infected CCR5-/- mice into infected CCR5-/- mice, and increased survival to 60%, the same as in infected CCR5+/+ control mice. We conclude that CCR5 is a critical antiviral and survival determinant in WNV infection of mice that acts by regulating trafficking of leukocytes to the infected brain.
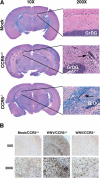
Figures





References
-
- Smithburn, K.C., T.P. Hughes, A.W. Burke, and J.H. Paul. 1940. Neurotropic virus isolated from the blood of a native of Uganda. Am. J. Trop. Med. 20:471–472.
-
- Lanciotti, R.S., J.T. Roehrig, V. Deubel, J. Smith, M. Parker, K. Steele, B. Crise, K.E. Volpe, M.B. Crabtree, J.H. Scherret, et al. 1999. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science. 286:2333–2337. - PubMed
-
- Centers for Disease Control and Prevention. 2004. West Nile virus activity–United States, October 6-12, 2004. MMWR Morb. Mortal. Wkly. Rep. 53:950–951. - PubMed
-
- van der Poel, W.H. 1999. West Nile-like virus is the cause of encephalitis in humans and horses and the death of hundreds of birds in New York. [In Dutch.] Tijdschr. Diergeneeskd. 124:704–705 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

