A histone code in meiosis: the histone kinase, NHK-1, is required for proper chromosomal architecture in Drosophila oocytes
- PMID: 16230526
- PMCID: PMC1276731
- DOI: 10.1101/gad.1348905
A histone code in meiosis: the histone kinase, NHK-1, is required for proper chromosomal architecture in Drosophila oocytes
Abstract
To promote faithful propagation of the genetic material during sexual reproduction, meiotic chromosomes undergo specialized morphological changes that ensure accurate segregation of homologous chromosomes. The molecular mechanisms that establish the meiotic chromosomal structures are largely unknown. We describe a mutation in a recently identified Histone H2A kinase, nhk-1, in Drosophila that leads to female sterility due to defects in the formation of the meiotic chromosomal structures. The metaphase I arrest and the karyosome, a critical prophase I chromosomal structure, require nucleosomal histone kinase-1 (NHK-1) function. The defects are a result of failure to disassemble the synaptonemal complex and to load condensin onto the mutant chromosomes. Embryos laid by nhk-1-/- mutant females arrest with aberrant polar bodies and mitotic spindles, revealing that mitosis is affected as well. We analyzed the role of Histone H2A phosphorylation with respect to the histone code hypothesis and found that it is required for acetylation of Histone H3 and Histone H4 in meiosis. These studies reveal a critical role for histone modifications in chromosome dynamics in meiosis and mitosis.
Figures


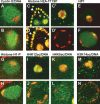
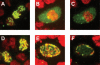
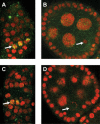
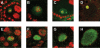
Similar articles
-
NHK-1 phosphorylates BAF to allow karyosome formation in the Drosophila oocyte nucleus.J Cell Biol. 2007 Dec 3;179(5):817-24. doi: 10.1083/jcb.200706067. Epub 2007 Nov 26. J Cell Biol. 2007. PMID: 18039935 Free PMC article.
-
The meiotic recombination checkpoint suppresses NHK-1 kinase to prevent reorganisation of the oocyte nucleus in Drosophila.PLoS Genet. 2010 Oct 28;6(10):e1001179. doi: 10.1371/journal.pgen.1001179. PLoS Genet. 2010. PMID: 21060809 Free PMC article.
-
Mutations in the chromosomal passenger complex and the condensin complex differentially affect synaptonemal complex disassembly and metaphase I configuration in Drosophila female meiosis.Genetics. 2009 Mar;181(3):875-87. doi: 10.1534/genetics.108.097741. Epub 2008 Dec 22. Genetics. 2009. PMID: 19104074 Free PMC article.
-
Histone modifications and the chromatin scaffold for meiotic chromosome architecture.Cell Cycle. 2006 Sep;5(18):2064-71. doi: 10.4161/cc.5.18.3253. Epub 2006 Sep 15. Cell Cycle. 2006. PMID: 16969105 Review.
-
The chromosomal basis of meiotic acentrosomal spindle assembly and function in oocytes.Chromosoma. 2017 Jun;126(3):351-364. doi: 10.1007/s00412-016-0618-1. Epub 2016 Nov 11. Chromosoma. 2017. PMID: 27837282 Free PMC article. Review.
Cited by
-
Regulating the construction and demolition of the synaptonemal complex.Nat Struct Mol Biol. 2016 May 4;23(5):369-77. doi: 10.1038/nsmb.3208. Nat Struct Mol Biol. 2016. PMID: 27142324 Review.
-
Bällchen is required for self-renewal of germline stem cells in Drosophila melanogaster.Biol Open. 2014 May 29;3(6):510-21. doi: 10.1242/bio.20147690. Biol Open. 2014. PMID: 24876388 Free PMC article.
-
Phosphorylation of the histone H3.3 variant in mitosis and meiosis of the urochordate Oikopleura dioica.Chromosome Res. 2007;15(2):189-201. doi: 10.1007/s10577-006-1112-z. Epub 2007 Feb 15. Chromosome Res. 2007. PMID: 17333540
-
Bällchen participates in proliferation control and prevents the differentiation of Drosophila melanogaster neuronal stem cells.Biol Open. 2014 Sep 4;3(10):881-6. doi: 10.1242/bio.20148631. Biol Open. 2014. PMID: 25190057 Free PMC article.
-
NHK-1 phosphorylates BAF to allow karyosome formation in the Drosophila oocyte nucleus.J Cell Biol. 2007 Dec 3;179(5):817-24. doi: 10.1083/jcb.200706067. Epub 2007 Nov 26. J Cell Biol. 2007. PMID: 18039935 Free PMC article.
References
-
- Ashburner M. 1989. Drosophila. A laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
-
- Belmont A.S., Braunfeld, M.B., Sedat, J.W., and Agard, D.A. 1989. Large-scale chromatin structural domains within mitotic and interphase chromosomes in vivo and in vitro. Chromosoma 98: 129-143. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
