The microtubule plus-end-tracking protein CLIP-170 associates with the spermatid manchette and is essential for spermatogenesis
- PMID: 16230537
- PMCID: PMC1257404
- DOI: 10.1101/gad.344505
The microtubule plus-end-tracking protein CLIP-170 associates with the spermatid manchette and is essential for spermatogenesis
Abstract
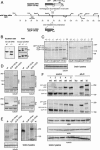
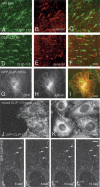
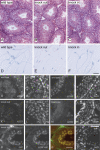
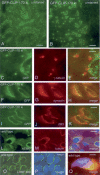
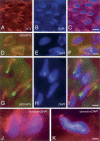
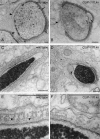
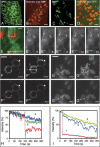
CLIP-170 is a microtubule "plus-end-tracking protein" implicated in the control of microtubule dynamics, dynactin localization, and the linking of endosomes to microtubules. To investigate the function of mouse CLIP-170, we generated CLIP-170 knockout and GFP-CLIP-170 knock-in alleles. Residual CLIP-170 is detected in lungs and embryos of homozygous CLIP-170 knockout mice, but not in other tissues and cell types, indicating that we have generated a hypomorphic mutant. Homozygous CLIP-170 knockout mice are viable and appear normal. However, male knockout mice are subfertile and produce sperm with abnormal heads. Using the knock-in mice, we followed GFP-CLIP-170 expression and behavior in dissected, live testis tubules. We detect plus-end-tracking GFP-CLIP-170 in spermatogonia. As spermatogenesis proceeds, GFP-CLIP-170 expression increases and the fusion protein strongly marks syncytia of differentiated spermatogonia and early prophase spermatocytes. Subsequently GFP-CLIP-170 levels drop, but during spermiogenesis (post-meiotic development), GFP-CLIP-170 accumulates again and is present on spermatid manchettes and centrosomes. Bleaching studies show that, as spermatogenesis progresses, GFP-CLIP-170 converts from a mobile plus-end-tracking protein to a relatively immobile protein. We propose that CLIP-170 has a structural function in the male germline, in particular in spermatid differentiation and sperm head shaping.
Figures

Similar articles
-
RIM-BP3 is a manchette-associated protein essential for spermiogenesis.Development. 2009 Feb;136(3):373-82. doi: 10.1242/dev.030858. Epub 2008 Dec 17. Development. 2009. PMID: 19091768
-
Ran, a GTP-binding protein involved in nucleocytoplasmic transport and microtubule nucleation, relocates from the manchette to the centrosome region during rat spermiogenesis.Mol Reprod Dev. 2002 Sep;63(1):131-40. doi: 10.1002/mrd.10164. Mol Reprod Dev. 2002. PMID: 12211070
-
CLIP-50 immunolocalization during mouse spermiogenesis suggests a role in shaping the sperm nucleus.Dev Biol. 2001 Aug 15;236(2):400-10. doi: 10.1006/dbio.2001.0286. Dev Biol. 2001. PMID: 11476580
-
Intramanchette transport (IMT): managing the making of the spermatid head, centrosome, and tail.Mol Reprod Dev. 2002 Sep;63(1):1-4. doi: 10.1002/mrd.10179. Mol Reprod Dev. 2002. PMID: 12211054 Review.
-
Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 2: changes in spermatid organelles associated with development of spermatozoa.Microsc Res Tech. 2010 Apr;73(4):279-319. doi: 10.1002/jemt.20787. Microsc Res Tech. 2010. PMID: 19941292 Review.
Cited by
-
CLASP2-dependent microtubule capture at the neuromuscular junction membrane requires LL5β and actin for focal delivery of acetylcholine receptor vesicles.Mol Biol Cell. 2015 Mar 1;26(5):938-51. doi: 10.1091/mbc.E14-06-1158. Epub 2015 Jan 14. Mol Biol Cell. 2015. PMID: 25589673 Free PMC article.
-
Is toxicant-induced Sertoli cell injury in vitro a useful model to study molecular mechanisms in spermatogenesis?Semin Cell Dev Biol. 2016 Nov;59:141-156. doi: 10.1016/j.semcdb.2016.01.003. Epub 2016 Jan 15. Semin Cell Dev Biol. 2016. PMID: 26779951 Free PMC article. Review.
-
α-tubulin tail modifications regulate microtubule stability through selective effector recruitment, not changes in intrinsic polymer dynamics.Dev Cell. 2021 Jul 26;56(14):2016-2028.e4. doi: 10.1016/j.devcel.2021.05.005. Epub 2021 May 21. Dev Cell. 2021. PMID: 34022132 Free PMC article.
-
Hypobaric hypoxia and reoxygenation induce proteomic profile changes in the rat brain cortex.Neuromolecular Med. 2013 Mar;15(1):82-94. doi: 10.1007/s12017-012-8197-7. Epub 2012 Sep 8. Neuromolecular Med. 2013. PMID: 22961459
-
Autonomous transposons tune their sequences to ensure somatic suppression.Nature. 2024 Feb;626(8001):1116-1124. doi: 10.1038/s41586-024-07081-0. Epub 2024 Feb 14. Nature. 2024. PMID: 38355802 Free PMC article.
References
-
- Akhmanova A. and Hoogenraad, C.C. 2005. Microtubule plus-end-tracking proteins: Mechanisms and functions. Curr. Opin. Cell Biol. 17: 47-54. - PubMed
-
- Akhmanova A., Hoogenraad, C.C., Drabek, K., Stepanova, T., Dortland, B., Verkerk, T., Vermeulen, W., Burgering, B.M., De Zeeuw, C.I., Grosveld, F., et al. 2001. Clasps are CLIP-115 and -170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts. Cell 104: 923-935. - PubMed
-
- Arnal I., Heichette, C., Diamantopoulos, G.S., and Chretien, D. 2004. CLIP-170/tubulin-curved oligomers coassemble at microtubule ends and promote rescues. Curr. Biol. 14: 2086-2095. - PubMed
-
- Bilbe G., Delabie, J., Bruggen, J., Richener, H., Asselbergs, F.A., Cerletti, N., Sorg, C., Odink, K., Tarcsay, L., Wiesendanger, W., et al. 1992. Restin: A novel intermediate filament-associated protein highly expressed in the Reed-Sternberg cells of Hodgkin's disease. EMBO J. 11: 2103-2113. - PMC - PubMed
-
- Brunner D. and Nurse, P. 2000. CLIP170-like tip1p spatially organizes microtubular dynamics in fission yeast. Cell 102: 695-704. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
