Insulin-like growth factors 1 and 2 induce lymphangiogenesis in vivo
- PMID: 16230630
- PMCID: PMC1266150
- DOI: 10.1073/pnas.0507865102
Insulin-like growth factors 1 and 2 induce lymphangiogenesis in vivo
Abstract
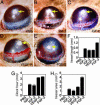
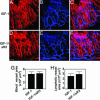
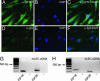
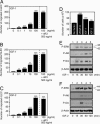
Lymphangiogenesis is an important process that contributes to the spread of cancer. Here we show that insulin-like growth factors 1 (IGF-1) and 2 (IGF-2) induce lymphangiogenesis in vivo. In a mouse cornea assay, IGF-1 and IGF-2 induce lymphangiogenesis as detected with LYVE-1, a specific marker for lymphatic endothelium. Interestingly, IGF-1-induced lymphangiogenesis could not be blocked by a soluble vascular endothelial growth factor receptor 3, suggesting that the vascular endothelial growth factor receptor 3-signaling pathway is not required for IGF-induced lymphangiogenesis. In vitro, IGF-1 and IGF-2 significantly stimulated proliferation and migration of primary lymphatic endothelial cells. IGF-1 and IGF-2 induced phosphorylation of intracellular signaling components, such as Akt, Src, and extracellular signal-regulated kinase in lymphatic endothelial cells. Immunohistochemistry, RT-PCR, and Affymetrix GeneChip microarray analysis showed that the receptors for IGFs are present in lymphatic endothelium. Together, our findings suggest that IGFs might act as direct lymphangiogenic factors, although any indirect roles in the induction of lymphangiogenesis cannot be excluded. Because members of the IGF ligand and receptor families are widely expressed in various types of solid tumors, our findings suggest that these factors are likely to contribute to lymphatic metastasis.
Figures

References
-
- Fidler, I. J. (2003) Nat. Rev. Cancer 3, 453–458. - PubMed
-
- Beasley, N. J., Prevo, R., Banerji, S., Leek, R. D., Moore, J., van Trappen, P., Cox, G., Harris, A. L. & Jackson, D. G. (2002) Cancer Res. 62, 1315–1320. - PubMed
-
- Dadras, S. S., Lange-Asschenfeldt, B., Velasco, P., Nguyen, L., Vora, A., Muzikansky, A., Jahnke, K., Hauschild, A., Hirakawa, S., Mihm, M. C. & Detmar, M. (2005) Mod. Pathol. 18, 1232–1242. - PubMed
-
- Alitalo, K. & Carmeliet, P. (2002) Cancer Cell 1, 219–227. - PubMed
-
- Cao, R., Bjorndahl, M. A., Religa, P., Clasper, S., Garvin, S., Galter, D., Meister, B., Ikomi, F., Tritsaris, K., Dissing, S., et al. (2004) Cancer Cell 6, 333–345. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

