Nongenomic responses to 17beta-estradiol in male rat mesenteric arteries abolish intrinsic gender differences in vascular responses
- PMID: 16231002
- PMCID: PMC1751239
- DOI: 10.1038/sj.bjp.0706422
Nongenomic responses to 17beta-estradiol in male rat mesenteric arteries abolish intrinsic gender differences in vascular responses
Abstract
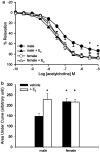
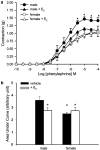
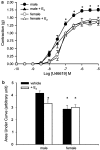
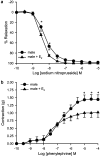
The aim of the present study was to investigate the gender differences in the acute effects of 17beta-estradiol on the rat superior mesenteric artery. Isometric tension was measured in rings of mesenteric arteries from both male and female Sprague-Dawley rats. Relaxation to acetylcholine was not significantly different between arteries (with endothelium) from male and female rats in the absence or presence of 17beta-estradiol. After blockade of endothelium-dependent hyperpolarizations with apamin (0.3 microM) plus charybdotoxin (0.1 microM), acute exposure to 17beta-estradiol (1 nM) for 30 min resulted in enhancement of relaxation to acetylcholine in arteries from male but not female rats. After acute exposure to 17beta-estradiol, mesenteric arteries from male rats were more sensitive to sodium nitroprusside than arteries from female rats. Contractions of mesenteric arteries to phenylephrine and 9,11-dideoxy-11alpha,9alpha-epoxymethanoprostaglandin F(2alpha) (U46619) were greater in arteries from male rats than female rats. This difference was not detected after acute exposure to 17beta-estradiol. In preparations without endothelium, the enhancement of relaxation and reduction in contraction in arteries from male rats were preserved. These results suggest that there exists a gender difference in the response to the acute nongenomic modulatory effect of 17beta-estradiol in rat mesenteric arteries. Arteries from male rats seem to be more sensitive to the modulatory effects of 17beta-estradiol than arteries from female rats. The effect appears to be mainly at the level of the vascular smooth muscles.
Figures



References
-
- AKISHITA M., KOZAKI K., ETO M., YOSHIZUMI M., ISHIKAWA M., TOBA K., ORIMO H., OUCHI Y. Estrogen attenuates endothelin-1 production by bovine endothelial cells via estrogen receptor. Biochem. Biophys. Res. Commun. 1998;251:17–21. - PubMed
-
- ASANO K., MARUYAMA S., USUI T., FUJIMOTO N. Regulation of estrongen receptor alpha and beta expression by testosterone in the rat prostate gland. Endocr. J. 2003;50:281–287. - PubMed
-
- BAUERSACHS J., POPP R., HECKER M., SAUER E., FLEMING I., BUSSE R. Nitric oxide attenuates the release of endothelium-derived hyperpolarizing factor. Circulation. 1996;94:3341–3347. - PubMed
-
- CREWS J.K., KHALIL R.A. Gender-specific inhibition of Ca2+ entry mechanisms of arterial vasoconstriction by sex hormones. Clin. Exp. Pharmacol. Physiol. 1999;26:707–715. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

