Analysis of macroscopic ionic currents mediated by GABArho1 receptors during lanthanide modulation predicts novel states controlling channel gating
- PMID: 16231008
- PMCID: PMC1751227
- DOI: 10.1038/sj.bjp.0706411
Analysis of macroscopic ionic currents mediated by GABArho1 receptors during lanthanide modulation predicts novel states controlling channel gating
Abstract
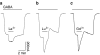
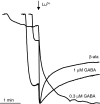
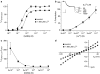
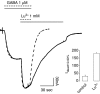
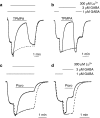
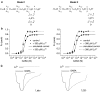
Lanthanide-induced modulation of GABA(C) receptors expressed in Xenopus oocytes was studied. We obtained two-electrode voltage-clamp recordings of ionic currents mediated by recombinant homomeric GABArho(1) receptors and performed numerical simulations of kinetic models of the macroscopic ionic currents.GABA-evoked chloride currents were potentiated by La(3+), Lu(3+) and Gd(3+) in the micromolar range. Lanthanide effects were rapid, reversible and voltage independent. The degree of potentiation was reduced by increasing GABA concentration.Lu(3+) also induced receptor desensitization and decreased the deactivation rate of GABArho(1) currents. In the presence of 300 microM Lu(3+), dose-response curves for GABA-evoked currents showed a significant enhancement of the maximum amplitude and an increase of the apparent affinity. The rate of onset of TPMPA and picrotoxin antagonism of GABArho(1) receptors was modulated by Lu(3+). These results suggest that the potentiation of the anionic current was the result of a direct lanthanide-receptor interaction at a site capable of allosterically modulating channel properties. Based on kinetic schemes, which included a second open state and a nonconducting desensitized state that closely reproduced the experimental results, two nonexclusive probable models of GABArho(1) channels gating are proposed.
Figures
Similar articles
-
Dopamine and serotonin modulate human GABAρ1 receptors expressed in Xenopus laevis oocytes.ACS Chem Neurosci. 2012 Feb 15;3(2):96-104. doi: 10.1021/cn200083m. Epub 2011 Dec 9. ACS Chem Neurosci. 2012. PMID: 22860179 Free PMC article.
-
Modulation of human GABArho1 receptors by taurine.Neurosci Res. 2008 Jul;61(3):302-8. doi: 10.1016/j.neures.2008.03.009. Epub 2008 Apr 4. Neurosci Res. 2008. PMID: 18479770
-
Redox modulation of homomeric rho1 GABA receptors.J Neurochem. 2008 Jun 1;105(6):2367-74. doi: 10.1111/j.1471-4159.2008.05319.x. J Neurochem. 2008. PMID: 18315569
-
Cerebral GABAA and GABAB receptors. Structure and function.Ann N Y Acad Sci. 1995 May 10;757:516-27. doi: 10.1111/j.1749-6632.1995.tb17511.x. Ann N Y Acad Sci. 1995. PMID: 7611709 Review. No abstract available.
-
Ionic basis of GABAA receptor channel function in the nervous system.Prog Neurobiol. 1994 Mar;42(4):489-537. doi: 10.1016/0301-0082(94)90049-3. Prog Neurobiol. 1994. PMID: 7522334 Review. No abstract available.
Cited by
-
An intracellular redox sensor for reactive oxygen species at the M3-M4 linker of GABAA ρ1 receptors.Br J Pharmacol. 2014 May;171(9):2291-9. doi: 10.1111/bph.12581. Br J Pharmacol. 2014. PMID: 24428763 Free PMC article.
-
Nitric oxide potentiation of the homomeric ρ1 GABA(C) receptor function.Br J Pharmacol. 2012 Nov;167(6):1369-77. doi: 10.1111/j.1476-5381.2012.02087.x. Br J Pharmacol. 2012. PMID: 22747884 Free PMC article.
-
An Update on GABAρ Receptors.Curr Neuropharmacol. 2010 Dec;8(4):422-33. doi: 10.2174/157015910793358141. Curr Neuropharmacol. 2010. PMID: 21629448 Free PMC article.
-
Dopamine and serotonin modulate human GABAρ1 receptors expressed in Xenopus laevis oocytes.ACS Chem Neurosci. 2012 Feb 15;3(2):96-104. doi: 10.1021/cn200083m. Epub 2011 Dec 9. ACS Chem Neurosci. 2012. PMID: 22860179 Free PMC article.
-
Allosteric modulation of retinal GABA receptors by ascorbic acid.J Neurosci. 2011 Jun 29;31(26):9672-82. doi: 10.1523/JNEUROSCI.5157-10.2011. J Neurosci. 2011. PMID: 21715633 Free PMC article.
References
-
- ACUNA-CASTILLO C., MORALES B., HUIDOBRO-TORO J.P. Zinc and copper modulate differentially the P2X4 receptor. J. Neurochem. 2000;74:1529–1537. - PubMed
-
- ARAKAWA O., NAKAHIRO M., NARAHASHI T. Mercury modulation of GABA-activated chloride channels and non-specific cation channels in rat dorsal root ganglion neurons. Brain Res. 1991;551:58–63. - PubMed
-
- CALVO D.J., MILEDI R. Activation of GABA rho 1 receptors by glycine and beta-alanine. Neuroreport. 1995;6:1118–1120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

