Activity of recombinant trypsin isoforms on human proteinase-activated receptors (PAR): mesotrypsin cannot activate epithelial PAR-1, -2, but weakly activates brain PAR-1
- PMID: 16231009
- PMCID: PMC1751236
- DOI: 10.1038/sj.bjp.0706410
Activity of recombinant trypsin isoforms on human proteinase-activated receptors (PAR): mesotrypsin cannot activate epithelial PAR-1, -2, but weakly activates brain PAR-1
Abstract
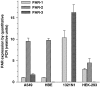
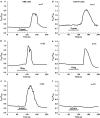
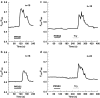
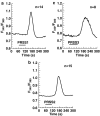
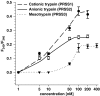
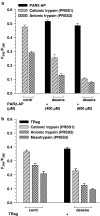
Trypsin-like serine proteinases trigger signal transduction pathways through proteolytic cleavage of proteinase-activated receptors (PARs) in many tissues. Three members, PAR-1, PAR-2 and PAR-4, are trypsin substrates, as trypsinolytic cleavage of the extracellular N terminus produces receptor activation. Here, the ability of the three human pancreatic trypsin isoforms (cationic trypsin, anionic trypsin and mesotrypsin (trypsin IV)) as recombinant proteins was tested on PARs. Using fura 2 [Ca(2+)](i) measurements, we analyzed three human epithelial cell lines, HBE (human bronchial epithelial), A549 (human pulmonary epithelial) and HEK (human embryonic kidney)-293 cells, which express functional PAR-1 and PAR-2. Human mesotrypsin failed to induce a PAR-mediated Ca(2+) response in human epithelial cells even at high concentrations. In addition, mesotrypsin did not affect the magnitude of PAR activation by subsequently added bovine trypsin. In HBE cells, which like A549 cells express high PAR-2 levels with negligible PAR-1 levels (<11%), half-maximal responses were seen for both cationic and anionic trypsins at about 5 nM. In the epithelial cells, mesotrypsin did not activate PAR-2 or PAR-1, whereas both anionic and cationic trypsins were comparable activators. We also investigated human astrocytoma 1321N1cells, which express PAR-1 and some PAR-3, but no PAR-2. High concentrations (>100 nM) of mesotrypsin produced a relatively weak Ca(2+) signal, apparently through PAR-1 activation. Half-maximal responses were observed at 60 nM mesotrypsin, and at 10-20 nM cationic and anionic trypsins. Using a desensitization assay with PAR-2-AP, we confirmed that both cationic and anionic trypsin isoforms cause [Ca(2+)](i) elevation in HBE cells mainly through PAR-2 activation. Desensitization of PAR-1 with thrombin receptor agonist peptide in 1321N1 cells demonstrated that all three recombinant trypsin isoforms act through PAR-1.Thus, the activity of human cationic and anionic trypsins on PARs was comparable to that of bovine pancreatic trypsin. Mesotrypsin (trypsin IV), in contrast to cationic and anionic trypsin, cannot activate or disable PARs in human epithelial cells, demonstrating that the receptors are no substrates for this isoenzyme. On the other hand, mesotrypsin activates PAR-1 in human astrocytoma cells. This might play a role in protection/degeneration or plasticity processes in the human brain.
Figures
Similar articles
-
Mesotrypsin, a brain trypsin, activates selectively proteinase-activated receptor-1, but not proteinase-activated receptor-2, in rat astrocytes.J Neurochem. 2006 Nov;99(3):759-69. doi: 10.1111/j.1471-4159.2006.04105.x. Epub 2006 Aug 11. J Neurochem. 2006. PMID: 16903872
-
Trypsin IV or mesotrypsin and p23 cleave protease-activated receptors 1 and 2 to induce inflammation and hyperalgesia.J Biol Chem. 2007 Sep 7;282(36):26089-100. doi: 10.1074/jbc.M703840200. Epub 2007 Jul 10. J Biol Chem. 2007. PMID: 17623652
-
Proteinase-activated receptor-2 and human lung epithelial cells: disarming by neutrophil serine proteinases.Am J Respir Cell Mol Biol. 2003 Mar;28(3):339-46. doi: 10.1165/rcmb.4908. Am J Respir Cell Mol Biol. 2003. PMID: 12594060
-
Review article: proteinase-activated receptors - novel signals for gastrointestinal pathophysiology.Aliment Pharmacol Ther. 2000 Mar;14(3):257-66. doi: 10.1046/j.1365-2036.2000.00690.x. Aliment Pharmacol Ther. 2000. PMID: 10735917 Review.
-
Biochemical and structural insights into mesotrypsin: an unusual human trypsin.Int J Biochem Mol Biol. 2013 Sep 13;4(3):129-39. Int J Biochem Mol Biol. 2013. PMID: 24049668 Free PMC article. Review.
Cited by
-
Unlocking the Promise of Decellularized Pancreatic Tissue: A Novel Approach to Support Angiogenesis in Engineered Tissue.Bioengineering (Basel). 2024 Feb 14;11(2):183. doi: 10.3390/bioengineering11020183. Bioengineering (Basel). 2024. PMID: 38391669 Free PMC article.
-
Small molecule inhibitors of mesotrypsin from a structure-based docking screen.PLoS One. 2017 May 2;12(5):e0176694. doi: 10.1371/journal.pone.0176694. eCollection 2017. PLoS One. 2017. PMID: 28463992 Free PMC article.
-
Pulmonary epithelium is a prominent source of proteinase-activated receptor-1-inducible CCL2 in pulmonary fibrosis.Am J Respir Crit Care Med. 2009 Mar 1;179(5):414-25. doi: 10.1164/rccm.200712-1827OC. Epub 2008 Dec 5. Am J Respir Crit Care Med. 2009. PMID: 19060230 Free PMC article.
-
PRSS3/mesotrypsin is a therapeutic target for metastatic prostate cancer.Mol Cancer Res. 2012 Dec;10(12):1555-66. doi: 10.1158/1541-7786.MCR-12-0314. Mol Cancer Res. 2012. PMID: 23258495 Free PMC article.
-
Human mesotrypsin exhibits restricted S1' subsite specificity with a strong preference for small polar side chains.FEBS J. 2006 Jul;273(13):2942-54. doi: 10.1111/j.1742-4658.2006.05305.x. Epub 2006 Jun 5. FEBS J. 2006. PMID: 16759229 Free PMC article.
References
-
- BLACKHART B.D., EMILSSON K., NGUYEN D., TENG W., MARTELLI A.J., NYSTEDT S., SUNDELIN J., SCARBOROUGH R.M. Ligand cross-reactivity within the protease-activated receptor family. J. Biol. Chem. 1996;271:16466–16471. - PubMed
-
- COMPTON S.J., CAIRNS J.A., PALMER K.J., AL-ANI B., HOLLENBERG M.D., WALLS A.F. A polymorphic protease-activated receptor 2 (PAR2) displaying reduced sensitivity to trypsin and differential responses to PAR agonists. J. Biol. Chem. 2000;275:39207–39212. - PubMed
-
- COTTRELL G.S., AMADESI S., GRADY E.F., BUNNETT N.W. Trypsin IV: A novel agonist of protease-activated receptors 2 and 4. J. Biol. Chem. 2004;279:13532–13539. - PubMed
-
- DERY O., CORVERA C.U., STEINHOFF M., BUNNETT N.W. Proteinase-activated receptors: novel mechanisms of signaling by serine proteases. Am. J. Physiol. 1998;274:C1429–C1452. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

