Wave-like spread of Ebola Zaire
- PMID: 16231972
- PMCID: PMC1262627
- DOI: 10.1371/journal.pbio.0030371
Wave-like spread of Ebola Zaire
Abstract
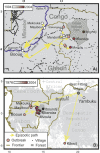
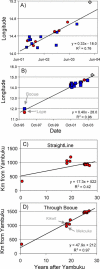
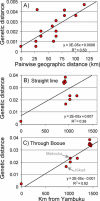
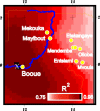
In the past decade the Zaire strain of Ebola virus (ZEBOV) has emerged repeatedly into human populations in central Africa and caused massive die-offs of gorillas and chimpanzees. We tested the view that emergence events are independent and caused by ZEBOV variants that have been long resident at each locality. Phylogenetic analyses place the earliest known outbreak at Yambuku, Democratic Republic of Congo, very near to the root of the ZEBOV tree, suggesting that viruses causing all other known outbreaks evolved from a Yambuku-like virus after 1976. The tendency for earlier outbreaks to be directly ancestral to later outbreaks suggests that outbreaks are epidemiologically linked and may have occurred at the front of an advancing wave. While the ladder-like phylogenetic structure could also bear the signature of positive selection, our statistical power is too weak to reach a conclusion in this regard. Distances among outbreaks indicate a spread rate of about 50 km per year that remains consistent across spatial scales. Viral evolution is clocklike, and sequences show a high level of small-scale spatial structure. Genetic similarity decays with distance at roughly the same rate at all spatial scales. Our analyses suggest that ZEBOV has recently spread across the region rather than being long persistent at each outbreak locality. Controlling the impact of Ebola on wild apes and human populations may be more feasible than previously recognized.
Figures


References
-
- Georges AJ, Leroy EM, Renaut AA, Benissan CT, Nabias RJ, et al. Ebola hemorrhagic fever outbreaks in Gabon, 1994–1997: Epidemiologic and health control issues. J Infect Dis. 1999;179:S65–S75. - PubMed
-
- Leroy EM, Rouquet P, Formenty P, Souquiere S, Kilbourne A, et al. Multiple Ebola virus transmission events and rapid decline of central African wildlife. Science. 2004;303:387–390. - PubMed
-
- Huijbregts B, De Wachter P, Obiang LSN, Akou ME. Ebola and the decline of gorilla Gorilla gorilla and chimpanzee Pan troglodytes populations in Minkebe Forest, north-eastern Gabon. Oryx. 2003;37:437–443.
-
- Walsh PD, Abernethy KA, Bermejo M, Beyers R, De Wachter P, et al. Catastrophic ape decline in western equatorial Africa. Nature. 2003;422:611–614. - PubMed
-
- Tutin CEG, Fernandez M. Nationwide census of gorilla (Gorilla gorilla) and chimpanzee (Pan troglodytes) populations in Gabon. Am J Primatol. 1984;6:313–336. - PubMed
Publication types
MeSH terms
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

