Intracellular composition of fatty acid affects the processing and function of tyrosinase through the ubiquitin-proteasome pathway
- PMID: 16232122
- PMCID: PMC1386001
- DOI: 10.1042/BJ20051419
Intracellular composition of fatty acid affects the processing and function of tyrosinase through the ubiquitin-proteasome pathway
Abstract
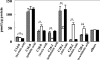
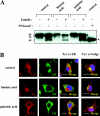
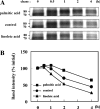
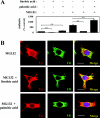
Proteasomes are multicatalytic proteinase complexes within cells that selectively degrade ubiquitinated proteins. We have recently demonstrated that fatty acids, major components of cell membranes, are able to regulate the proteasomal degradation of tyrosinase, a critical enzyme required for melanin biosynthesis, in contrasting manners by relative increases or decreases in the ubiquitinated tyrosinase. In the present study, we show that altering the intracellular composition of fatty acids affects the post-Golgi degradation of tyrosinase. Incubation with linoleic acid (C18:2) dramatically changed the fatty acid composition of cultured B16 melanoma cells, i.e. the remarkable increase in polyunsaturated fatty acids such as linoleic acid and arachidonic acid (C20:4) was compensated by the decrease in monounsaturated fatty acids such as oleic acid (C18:1) and palmitoleic acid (C16:1), with little effect on the proportion of saturated to unsaturated fatty acid. When the composition of intracellular fatty acids was altered, tyrosinase was rapidly processed to the Golgi apparatus from the ER (endoplasmic reticulum) and the degradation of tyrosinase was increased after its maturation in the Golgi. Retention of tyrosinase in the ER was observed when cells were treated with linoleic acid in the presence of proteasome inhibitors, explaining why melanin synthesis was decreased in cells treated with linoleic acid and a proteasome inhibitor despite the abrogation of tyrosinase degradation. These results suggest that the intracellular composition of fatty acid affects the processing and function of tyrosinase in connection with the ubiquitin-proteasome pathway and suggest that this might be a common physiological approach to regulate protein degradation.
Figures
References
-
- Chawla A., Repa J. J., Evans R. M., Mangelsdorf D. J. Nuclear receptors and lipid physiology: opening the X-files. Science. 2001;294:1866–1870. - PubMed
-
- Clarke S. D. The multi-dimensional regulation of gene expression by fatty acids: polyunsaturated fats as nutrient sensors. Curr. Opin. Lipidol. 2004;15:13–18. - PubMed
-
- Tahin Q. S., Blum M., Carafoli E. The fatty acid composition of subcellular membranes of rat liver, heart, and brain: diet-induced modifications. Eur. J. Biochem. 1981;121:5–13. - PubMed
-
- Awad A. B., Chattopadhyay J. P. Effect of dietary saturated fatty acids on intracellular free fatty acids and kinetic properties of hormone-sensitive lipase of rat adipocytes. J. Nutr. 1986;116:1095–1100. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

