Increase of CCL20 expression by human gingival fibroblasts upon stimulation with cytokines and bacterial endotoxin
- PMID: 16232215
- PMCID: PMC1809524
- DOI: 10.1111/j.1365-2249.2005.02912.x
Increase of CCL20 expression by human gingival fibroblasts upon stimulation with cytokines and bacterial endotoxin
Abstract
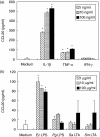
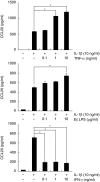
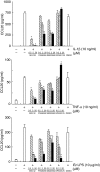
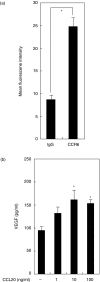
We have demonstrated recently that CCL20 was expressed in periodontal diseased tissues and abundant CCR6 positive T cells infiltrated in periodontally diseased tissue. However, it is uncertain which cells can elicit CCL20 production. In the present study, we examined the properties of CCL20 production by human gingival fibroblasts (HGF) culture. Here, we report that interleukin-1 beta (IL-1beta), tumour necrosis factor-alpha (TNF-alpha) and Escherichia coli lipopolysaccharide (LPS) can significantly induce the production of CCL20 by HGF. We found that TNF-alpha and E. coli LPS enhanced the production of CCL20 by HGF treated with IL-1beta. In contrast, interferon-gamma (IFN-gamma) dramatically diminished CCL20 production induced by IL-1beta. Moreover, we demonstrated that nuclear factor-kappaB (NF-kappaB), p38 mitogen-activated protein kinase (MAPK) and extracellular signal-regulated kinases (ERK) play an important role in mediating the production of CCL20 induced by IL-1beta and TNF-alpha. On the other hand, we found that not only NF-kappaB, p38 MAPK and ERK but also c-Jun NH2-terminal kinase (JNK) are involved in CCL20 production induced by E. coli LPS. Finally, we found that HGF express CCR6, CCL20 receptor, and CCL20 induced vascular endothelial growth factor (VEGF) by HGF. Taken together, these findings that HGF will be a source of CCL20 in periodontal tissue, and the CCL20 production will be controlled by proinflammatory cytokine and bacterial LPS in periodontally diseased tissue. Thus, CCL20 by HGF might be involved in inflammatory cells infiltration, and promote the progression of periodontal disease.
Figures

Similar articles
-
Proinflammatory effects of tumour necrosis factor-like weak inducer of apoptosis (TWEAK) on human gingival fibroblasts.Clin Exp Immunol. 2006 Dec;146(3):540-9. doi: 10.1111/j.1365-2249.2006.03233.x. Clin Exp Immunol. 2006. PMID: 17100776 Free PMC article.
-
CC chemokine ligand 17 in periodontal diseases: expression in diseased tissues and production by human gingival fibroblasts.J Periodontal Res. 2008 Aug;43(4):471-7. doi: 10.1111/j.1600-0765.2007.01080.x. J Periodontal Res. 2008. PMID: 18557811
-
CXC chemokine ligand 16 in periodontal diseases: expression in diseased tissues and production by cytokine-stimulated human gingival fibroblasts.Clin Exp Immunol. 2007 Jul;149(1):146-54. doi: 10.1111/j.1365-2249.2007.03398.x. Epub 2007 Apr 25. Clin Exp Immunol. 2007. PMID: 17459077 Free PMC article.
-
Perspective of cytokine regulation for periodontal treatment: fibroblast biology.J Periodontol. 2003 Jan;74(1):103-10. doi: 10.1902/jop.2003.74.1.103. J Periodontol. 2003. PMID: 12593604 Review.
-
Anti-inflammatory compounds of plant origin. Part II. modulation of pro-inflammatory cytokines, chemokines and adhesion molecules.Planta Med. 2004 Feb;70(2):93-103. doi: 10.1055/s-2004-815483. Planta Med. 2004. PMID: 14994184 Review.
Cited by
-
T4SS-dependent TLR5 activation by Helicobacter pylori infection.Nat Commun. 2019 Dec 16;10(1):5717. doi: 10.1038/s41467-019-13506-6. Nat Commun. 2019. PMID: 31844047 Free PMC article.
-
Effect of lipopolysaccharide on cell proliferation and vascular endothelial growth factor secretion of periodontal ligament stem cells.Saudi Dent J. 2020 Mar;32(3):148-154. doi: 10.1016/j.sdentj.2019.08.001. Epub 2019 Aug 23. Saudi Dent J. 2020. PMID: 32180672 Free PMC article.
-
Toll-Like Receptors and Dental Mesenchymal Stromal Cells.Front Oral Health. 2021 Apr 16;2:648901. doi: 10.3389/froh.2021.648901. eCollection 2021. Front Oral Health. 2021. PMID: 35048000 Free PMC article. Review.
-
Keratinocyte Growth Factor Stimulates Macrophage Inflammatory Protein 3α and Keratinocyte-derived Chemokine Secretion by Mouse Uterine Epithelial Cells.Am J Reprod Immunol. 2010 Sep;64(3):197-211. doi: 10.1111/j.1600-0897.2010.00850.x. Am J Reprod Immunol. 2010. PMID: 20455876 Free PMC article.
-
IL-22 enhances CCL20 production in IL-1β-stimulated human gingival fibroblasts.Inflammation. 2014 Dec;37(6):2062-6. doi: 10.1007/s10753-014-9939-5. Inflammation. 2014. PMID: 24902798
References
-
- Dzink JL, Tanner AC, Haffajee AD, Socransky SS. Gram negative species associated with active destructive periodontal lesions. J Clin Periodontol. 1985;12:648–59. - PubMed
-
- Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL., Jr Microbial complexes in subgingival plaque. J Clin Periodontol. 1998;25:134–44. - PubMed
-
- Taubman MA, Eastcott JW, Shimauchi H, Takeichi O, Smith DJ. Modulatory role of T lymphocytes in periodontal inflammation. In: Genco RJ, Hamada S, Mergenhagen SE, et al., editors. Molecular pathogenesis of periodontal disease. Washington, DC: American Society for Microbiology; 1994. p. 14757.
-
- Zehnder M, Greenspan JS, Greenspan D, Bickel M. Chemokine gene expression in human oral mucosa. Eur J Oral Sci. 1999;107:231–5. - PubMed
-
- Yu X, Graves DT. Fibroblasts, mononuclear phagocytes, and endothelial cells express monocyte chemoattractant protein-1 (MCP-1) in inflamed human gingiva. J Periodontol. 1995;66:80–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

