The effects of atorvastatin on gluten-induced intestinal T cell responses in coeliac disease
- PMID: 16232221
- PMCID: PMC1809523
- DOI: 10.1111/j.1365-2249.2005.02915.x
The effects of atorvastatin on gluten-induced intestinal T cell responses in coeliac disease
Abstract
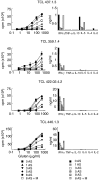
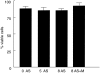
Various experimental models suggest that the cholesterol-lowering drugs statins may also modulate immune responses. Cellular level studies on human disorders are needed, however, to provide a rational basis for clinical testing of statins as immune therapy. Coeliac disease, a chronic small intestinal inflammation driven by HLA-DQ2 restricted mucosal T cells that are specific for ingested wheat gluten peptides, is in many ways ideal for this purpose. In addition, there is a need for alternative treatment to the gluten-free diet in this disorder. Here we have assessed the effects of atorvastatin on gluten-reactive T cells, dendritic cells and the coeliac mucosa by in vitro culture of biopsies. Atorvastatin inhibited gluten-induced proliferation and specific cytokine production of human intestinal gluten-reactive T cell clones and lines. Dendritic cells exposed to atorvastatin displayed a reduced expression of the costimulatory molecule CD83 upon maturation with lipopolysaccharide. Incubation of intestinal biopsy specimens with atorvastatin in vitro, however, did not influence gluten-induced cytokine release. In conclusion, atorvastatin has specific effects on isolated gluten-reactive T cells and dendritic cells, but does not shut down the gluten-induced production of proinflammatory cytokines in intestinal biopsies.
Figures



Similar articles
-
A unique dendritic cell subset accumulates in the celiac lesion and efficiently activates gluten-reactive T cells.Gastroenterology. 2006 Aug;131(2):428-38. doi: 10.1053/j.gastro.2006.06.002. Gastroenterology. 2006. PMID: 16890596
-
Evidence for the role of interferon-alfa production by dendritic cells in the Th1 response in celiac disease.Gastroenterology. 2007 Oct;133(4):1175-87. doi: 10.1053/j.gastro.2007.08.018. Epub 2007 Aug 14. Gastroenterology. 2007. PMID: 17919493
-
Gluten-free diet induces regression of T-cell activation in the rectal mucosa of patients with celiac disease.Am J Gastroenterol. 1998 Sep;93(9):1527-30. doi: 10.1111/j.1572-0241.1998.00439.x. Am J Gastroenterol. 1998. PMID: 9732937
-
Celiac disease: from pathogenesis to novel therapies.Gastroenterology. 2009 Dec;137(6):1912-33. doi: 10.1053/j.gastro.2009.09.008. Epub 2009 Sep 18. Gastroenterology. 2009. PMID: 19766641 Review.
-
Gluten intolerance (coeliac disease).Ann Allergy. 1984 Dec;53(6 Pt 2):637-42. Ann Allergy. 1984. PMID: 6391293 Review.
Cited by
-
Regulation of different inflammatory diseases by impacting the mevalonate pathway.Immunology. 2009 May;127(1):18-25. doi: 10.1111/j.1365-2567.2008.03011.x. Immunology. 2009. PMID: 19191903 Free PMC article. Review.
-
Rapid accumulation of CD14+CD11c+ dendritic cells in gut mucosa of celiac disease after in vivo gluten challenge.PLoS One. 2012;7(3):e33556. doi: 10.1371/journal.pone.0033556. Epub 2012 Mar 16. PLoS One. 2012. PMID: 22438948 Free PMC article.
References
-
- Nath N, Giri S, Prasad R, et al. Potential targets of 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor for multiple sclerosis therapy. J Immunol. 2004;172:1273–86. - PubMed
-
- Youssef S, Stuve O, Patarroyo JC, et al. The HMG-CoA reductase inhibitor, atorvastatin, promotes a Th2 bias and reverses paralysis in central nervous system autoimmune disease. Nature. 2002;420:78–84. - PubMed
-
- Leung BP, Sattar N, Crilly A, et al. A novel anti-inflammatory role for simvastatin in inflammatory arthritis. J Immunol. 2003;170:1524–30. - PubMed
-
- Kwak B, Mulhaupt F, Myit S, Mach F. Statins as a newly recognized type of immunomodulator. Nat Med. 2000;6:1399–402. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

