AML engraftment in the NOD/SCID assay reflects the outcome of AML: implications for our understanding of the heterogeneity of AML
- PMID: 16234360
- PMCID: PMC1895911
- DOI: 10.1182/blood-2005-06-2325
AML engraftment in the NOD/SCID assay reflects the outcome of AML: implications for our understanding of the heterogeneity of AML
Abstract
The nonobese diabetic/severe combined immunodeficient (NOD/SCID) assay is the current model for assessment of human normal and leukemic stem cells. We explored why 51% of 59 acute myeloid leukemia (AML) patients were unable to initiate leukemia in NOD/SCID mice. Increasing the cell dose, using more permissive recipients, and alternative tissue sources did not cause AML engraftment in most previously nonengrafting AML samples. Homing of AML cells to the marrow was the same between engrafters and nonengrafters. FLT3 internal tandem duplication (ITD) and nucleophosmin mutations occurred at a similar frequency in engrafters and nonengrafters. The only variable that was related to engraftment ability was the karyotypically defined risk stratification of individual AML cases. Of interest, follow-up of younger patients with intermediate-risk AML revealed a significant difference in overall survival between NOD/SCID engrafting and nonengrafting AMLs. Hence, the ability of AML to engraft in the NOD/SCID assay seems to be an inherent property of AML cells, independent of homing, conditioning, or cell frequency/source, which is directly related to prognosis. Our results suggest an important difference between leukemic initiating cells between engrafting and nonengrafting AML cases that correlates with treatment response.
Figures
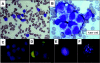

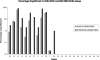
 ) and B2m-/- NOD/SCID (▪) mice in paired experiments. Six weeks later, bone marrow engraftment was assessed via flow cytometry. AML engraftment was recorded if human CD33+/CD45+ myeloid cells were present without an accompanying CD19+/CD45+ B-cell population. Ten of 12 AML cases that failed to engraft in the NOD/SCID assay did not engraft in the B2m-/- NOD/SCID model.
) and B2m-/- NOD/SCID (▪) mice in paired experiments. Six weeks later, bone marrow engraftment was assessed via flow cytometry. AML engraftment was recorded if human CD33+/CD45+ myeloid cells were present without an accompanying CD19+/CD45+ B-cell population. Ten of 12 AML cases that failed to engraft in the NOD/SCID assay did not engraft in the B2m-/- NOD/SCID model.
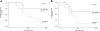
References
-
- Bhatia M, Bonnet D, Murdoch B, Gan OI, Dick JE. A newly discovered class of human hematopoietic cells with SCID-repopulating activity. Nat Med. 1998;4: 1038-1045. - PubMed
-
- Kollet O, Peled A, Byk T, et al. beta2 microglobulin-deficient (B2m(null)) NOD/SCID mice are excellent recipients for studying human stem cell function. Blood. 2000;95: 3102-3105. - PubMed
-
- Sutherland HJ, Blair A, Zapf RW. Characterization of a hierarchy in human acute myeloid leukemia progenitor cells. Blood. 1996;87: 4754-4761. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

