Glutamine depletion potentiates leucocyte-dependent inflammatory events induced by carrageenan or Clostridium difficile toxin A in rats
- PMID: 16236122
- PMCID: PMC1802418
- DOI: 10.1111/j.1365-2567.2005.02232.x
Glutamine depletion potentiates leucocyte-dependent inflammatory events induced by carrageenan or Clostridium difficile toxin A in rats
Abstract
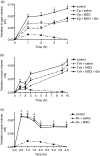
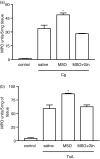
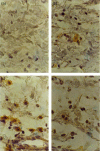
This research investigated the effect of glutamine (Gln) depletion on leucocyte-dependent inflammatory events. Rats were treated intraperitoneally, 16 hr prior to the peak of every parameter evaluated, with either 0.9% NaCl, methionine-sulphoximine (MSO, an inhibitor of endogenous Gln synthesis, 25 mg/kg) or with MSO + Gln (MSO as above plus Gln 3 g/kg in three doses). MSO-induced Gln depletion increased paw oedema induced both by carrageenan (Cg) and by Clostridium difficile toxin A (TxA) (66.2% and 45.5%, respectively; P < 0.05). In dextran-injected animals, oedema and myeloperoxidase (MPO) activity were not modified by Gln depletion. In Cg-treated paws, Gln depletion increased MPO activity by 44% (P < 0.05), interleukin-1beta (IL-1beta) and tumour necrosis factor-alpha (TNF-alpha) concentrations by 47% and 52%, respectively (P < 0.05), and immunostaining for TNF-alpha in paw tissue. In TxA-injected paws, Gln depletion increased MPO activity (46%; P < 0.05). Gln depletion increased Cg- and TxA-induced neutrophil migration to subcutaneous air pouches by 56% and 77% (P < 0.05), respectively, but did not affect migration induced by N-formyl-methionyl-leucyl-phenylalanine (fMLP). Gln infusions reversed all the effects of MSO. Leucocyte counts did not differ between groups. Gln depletion potentiates acute inflammation, possibly by increasing neutrophil migration through resident cell activation and production of IL-1beta and TNF-alpha. Gln supplementation reverses these effects and may be useful during inflammatory catabolic stress.
Figures


Similar articles
-
A study on the mechanisms involving the anti-inflammatory effect of amitriptyline in carrageenan-induced paw edema in rats.Eur J Pharmacol. 2011 Sep 30;667(1-3):396-401. doi: 10.1016/j.ejphar.2011.05.053. Epub 2011 Jun 1. Eur J Pharmacol. 2011. PMID: 21645506
-
Nitroxides attenuate carrageenan-induced inflammation in rat paws by reducing neutrophil infiltration and the resulting myeloperoxidase-mediated damage.Free Radic Biol Med. 2012 Nov 15;53(10):1942-53. doi: 10.1016/j.freeradbiomed.2012.09.001. Epub 2012 Sep 14. Free Radic Biol Med. 2012. PMID: 22982597
-
Clostridium difficile toxin A induces the release of neutrophil chemotactic factors from rat peritoneal macrophages: role of interleukin-1beta, tumor necrosis factor alpha, and leukotrienes.Infect Immun. 1997 Jul;65(7):2740-6. doi: 10.1128/iai.65.7.2740-2746.1997. Infect Immun. 1997. PMID: 9199444 Free PMC article.
-
Evaluation of the anti-inflammatory activity of riparin II (O-methil-N-2-hidroxi-benzoyl tyramine) in animal models.Chem Biol Interact. 2013 Oct 5;205(3):165-72. doi: 10.1016/j.cbi.2013.07.007. Epub 2013 Jul 17. Chem Biol Interact. 2013. PMID: 23872256
-
Antiinflammatory and antinociceptive effects in mice of a sulfated polysaccharide fraction extracted from the marine red algae Gracilaria caudata.Immunopharmacol Immunotoxicol. 2013 Feb;35(1):93-100. doi: 10.3109/08923973.2012.707211. Epub 2012 Jul 26. Immunopharmacol Immunotoxicol. 2013. PMID: 22830978
Cited by
-
The effects of acute oral glutamine supplementation on exercise-induced gastrointestinal permeability and heat shock protein expression in peripheral blood mononuclear cells.Cell Stress Chaperones. 2015 Jan;20(1):85-93. doi: 10.1007/s12192-014-0528-1. Epub 2014 Jul 26. Cell Stress Chaperones. 2015. PMID: 25062931 Free PMC article.
-
Host and Clostridioides difficile-Response Modulated by Micronutrients and Glutamine: An Overview.Front Nutr. 2022 Jun 20;9:849301. doi: 10.3389/fnut.2022.849301. eCollection 2022. Front Nutr. 2022. PMID: 35795588 Free PMC article. Review.
-
Intestinal epithelial restitution after TcdB challenge and recovery from Clostridium difficile infection in mice with alanyl-glutamine treatment.J Infect Dis. 2013 May 15;207(10):1505-15. doi: 10.1093/infdis/jit041. Epub 2013 Jan 28. J Infect Dis. 2013. PMID: 23359592 Free PMC article.
References
-
- Newsholme EA, Newsholme P, Curi R. The role of the citric acid cycle in cells of the immune system and its importance in sepsis, trauma and burns. Biochem Soc Symp. 1987;54:145–61. - PubMed
-
- Pithon-Curi TC, Pires-De-Melo M, De-Azevedo R, Zorn TMT, Curi R. Glutamine utilization by rat neutrophils. Presence of phosphate-dependent glutaminase. Am J Physiol. 1997;273:C1124–9. - PubMed
-
- Horig H, Spagnoli GC, Filgueira L, Babst R, Gallati H, Harder F, Juretic A, Heberer M. Exogenous glutamine requirement is confined to late events of T cell activation. J Cell Biochem. 1993;53:343–51. - PubMed
-
- Yaqoob P, Calder PC. Cytokine production by human peripheral blood mononuclear cells: differential senstivity to glutamine availability. Cytokine. 1998;10:790–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

