A multicenter trial of the efficacy and safety of tigecycline versus imipenem/cilastatin in patients with complicated intra-abdominal infections [Study ID Numbers: 3074A1-301-WW; ClinicalTrials.gov Identifier: NCT00081744]
- PMID: 16236177
- PMCID: PMC1277826
- DOI: 10.1186/1471-2334-5-88
A multicenter trial of the efficacy and safety of tigecycline versus imipenem/cilastatin in patients with complicated intra-abdominal infections [Study ID Numbers: 3074A1-301-WW; ClinicalTrials.gov Identifier: NCT00081744]
Abstract
Background: Complicated intra-abdominal infections (cIAI) remain challenging to treat because of their polymicrobial etiology including multi-drug resistant bacteria. The efficacy and safety of tigecycline, an expanded broad-spectrum glycylcycline antibiotic, was compared with imipenem/cilastatin (IMI/CIS) in patients with cIAI.
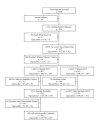
Methods: A prospective, double-blind, multinational trial was conducted in which patients with cIAI randomly received intravenous (IV) tigecycline (100 mg initial dose, then 50 mg every 12 hours [q12h]) or IV IMI/CIS (500/500 mg q6h or adjusted for renal dysfunction) for 5 to14 days. Clinical response at the test-of-cure (TOC) visit (14-35 days after therapy) for microbiologically evaluable (ME) and microbiological modified intent-to-treat (m-mITT) populations were the co-primary efficacy endpoint populations.
Results: A total of 825 patients received >or= 1 dose of study drug. The primary diagnoses for the ME group were complicated appendicitis (59%), and intestinal (8.8%) and gastric/duodenal perforations (4.6%). For the ME group, clinical cure rates at TOC were 80.6% (199/247) for tigecycline versus 82.4% (210/255) for IMI/CIS (95% CI -8.4, 5.1 for non-inferiority tigecycline versus IMI/CIS). Corresponding clinical cure rates within the m-mITT population were 73.5% (227/309) for tigecycline versus 78.2% (244/312) for IMI/CIS (95% CI -11.0, 2.5). Nausea (31.0% tigecycline, 24.8% IMI/CIS [P = 0.052]), vomiting (25.7% tigecycline, 19.4% IMI/CIS [P = 0.037]), and diarrhea (21.3% tigecycline, 18.9% IMI/CIS [P = 0.435]) were the most frequently reported adverse events.
Conclusion: This study demonstrates that tigecycline is as efficacious as imipenem/cilastatin in the treatment of patients with cIAI.
Trial registration: ClinicalTrials.gov NCT00081744.
Figures
References
-
- Solomkin JS, Mazuski JE, Baron EJ, Sawyer RG, Nathens AB, DiPiro JT, Buchman T, Dellinger EP, Jernigan J, Gorbach S, Chow AW, Bartlett J. Guidelines for the selection of anti-infective agents for complicated intra-abdominal infections. Clin Infect Dis. 2003;37:997–1005. doi: 10.1086/378702. - DOI - PubMed
-
- Gorbach SL. Intraabdominal infections. Clin Infect Dis. 1993;17:961–5. - PubMed
-
- Lorber B, Swenson RM. The bacteriology of intra-abdominal infections. Surg Clin North Am. 1975;55:1349–54. - PubMed
-
- Burnett RJ, Haverstock DC, Dellinger EP, Reinhart HH, Bohnen JM, Rotstein OD, Vogel SB, Solomkin JS. Definition of the role of enterococcus in intraabdominal infection: analysis of a prospective randomized trial. Surgery. 1995;118:716–21. - PubMed
-
- de Vera ME, Simmons RL. Antibiotic-resistant enterococci and the changing face of surgical infections. Arch Surg. 1996;131:338–42. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical