Negative regulation of Toll-like-receptor signaling by IRF-4
- PMID: 16236719
- PMCID: PMC1257749
- DOI: 10.1073/pnas.0508327102
Negative regulation of Toll-like-receptor signaling by IRF-4
Abstract
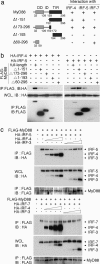
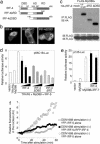
The recognition of microbial components by Toll-like receptors (TLRs) is an event central to the activation of innate and adaptive immune systems. TLR activation triggers the induction of downstream target genes, wherein the TLR-interacting adaptor molecule MyD88 recruits various signaling molecules and transcription factors. Two members of the IFN regulatory factor (IRF) family of transcription factors, IRF-5 and IRF-7, interact with MyD88 and induce proinflammatory cytokines and type I IFNs, respectively. Here, we show that IRF-4 also interacts with MyD88 and acts as a negative regulator of TLR signaling. IRF-4 mRNA is induced by TLR activation, and IRF-4 competes with IRF-5, but not with IRF-7, for MyD88 interaction. The TLR-dependent induction of proinflammatory cytokines is markedly enhanced in peritoneal macrophages from mice deficient in the Irf4 gene, whereas the induction is inhibited by the ectopic expression of IRF-4 in a macrophage cell line. The critical function of IRF-4 in TLR signaling in vivo is underscored by the observation that Irf4-deficient mice show hypersensitivity to DNA-induced shock, with elevated serum proinflammatory cytokine levels. This study may provide an insight into the complex regulatory mechanisms of MyD88 signaling by IRFs.
Figures




Similar articles
-
Integral role of IRF-5 in the gene induction programme activated by Toll-like receptors.Nature. 2005 Mar 10;434(7030):243-9. doi: 10.1038/nature03308. Epub 2005 Jan 23. Nature. 2005. PMID: 15665823
-
Evidence for licensing of IFN-gamma-induced IFN regulatory factor 1 transcription factor by MyD88 in Toll-like receptor-dependent gene induction program.Proc Natl Acad Sci U S A. 2006 Oct 10;103(41):15136-41. doi: 10.1073/pnas.0607181103. Epub 2006 Oct 3. Proc Natl Acad Sci U S A. 2006. PMID: 17018642 Free PMC article.
-
IRF-7 is the master regulator of type-I interferon-dependent immune responses.Nature. 2005 Apr 7;434(7034):772-7. doi: 10.1038/nature03464. Epub 2005 Mar 30. Nature. 2005. PMID: 15800576
-
TLR pathways and IFN-regulatory factors: to each its own.Eur J Immunol. 2007 Feb;37(2):306-9. doi: 10.1002/eji.200637009. Eur J Immunol. 2007. PMID: 17273997 Review.
-
Toll-like receptor signaling and IRF transcription factors.IUBMB Life. 2006 May-Jun;58(5-6):290-5. doi: 10.1080/15216540600702206. IUBMB Life. 2006. PMID: 16754320 Review.
Cited by
-
Systems Biological Approaches Reveal Non-additive Responses and Multiple Crosstalk Mechanisms between TLR and GPCR Signaling.Genomics Inform. 2012 Sep;10(3):153-66. doi: 10.5808/GI.2012.10.3.153. Epub 2012 Sep 28. Genomics Inform. 2012. PMID: 23166526 Free PMC article.
-
GM-CSF-Dependent Inflammatory Pathways.Front Immunol. 2019 Sep 4;10:2055. doi: 10.3389/fimmu.2019.02055. eCollection 2019. Front Immunol. 2019. PMID: 31552022 Free PMC article. Review.
-
Manipulation of the toll-like receptor 7 signaling pathway by Epstein-Barr virus.J Virol. 2007 Sep;81(18):9748-58. doi: 10.1128/JVI.01122-07. Epub 2007 Jul 3. J Virol. 2007. PMID: 17609264 Free PMC article.
-
IRF-4 and c-Rel expression in antiviral-resistant adult T-cell leukemia/lymphoma.Blood. 2007 Apr 1;109(7):3060-8. doi: 10.1182/blood-2006-07-036368. Blood. 2007. PMID: 17138822 Free PMC article.
-
GM-CSF in inflammation.J Exp Med. 2020 Jan 6;217(1):e20190945. doi: 10.1084/jem.20190945. J Exp Med. 2020. PMID: 31611249 Free PMC article. Review.
References
-
- Janeway, C. A., Jr., & Medzhitov, R. (2002) Annu. Rev. Immunol. 20, 197-216. - PubMed
-
- Akira, S. & Takeda, K. (2004) Nat. Rev. Immunol. 4, 499-511. - PubMed
-
- Medzhitov, R., Preston-Hurlburt, P., Kopp, E., Stadlen, A., Chen, C., Ghosh, S. & Janeway, C. A., Jr. (1998) Mol. Cell 2, 253-258. - PubMed
-
- Wesche, H., Henzel, W. J., Shillinglaw, W., Li, S. & Cao, Z. (1997) Immunity 7, 837-847. - PubMed
-
- Suzuki, N., Suzuki, S. & Yeh, W. C. (2002) Trends Immunol. 23, 503-506. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

