GMx33 associates with the trans-Golgi matrix in a dynamic manner and sorts within tubules exiting the Golgi
- PMID: 16236792
- PMCID: PMC1345686
- DOI: 10.1091/mbc.e05-07-0682
GMx33 associates with the trans-Golgi matrix in a dynamic manner and sorts within tubules exiting the Golgi
Abstract
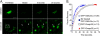
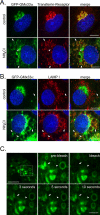
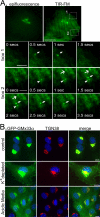
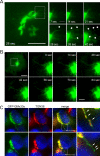
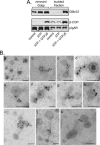
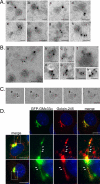
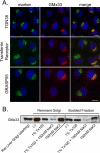
The trans-Golgi matrix consists of a group of proteins dynamically associated with the trans-Golgi and thought to be involved in anterograde and retrograde Golgi traffic, as well as interactions with the cytoskeleton and maintenance of the Golgi structure. GMx33 is localized to the cytoplasmic face of the trans-Golgi and is also present in a large cytoplasmic pool. Here we demonstrate that GMx33 is dynamically associated with the trans-Golgi matrix, associating and dissociating with the Golgi in seconds. GMx33 can be locked onto the trans-Golgi matrix by GTPgammaS, indicating that its association is regulated in a GTP-dependent manner like several other Golgi matrix proteins. Using live-cell imaging we show that GMx33 exits the Golgi associated with tubules and within these tubules GMx33 segregates from transmembrane proteins followed by fragmentation of the tubules into smaller tubules and vesicles. Within vesicles produced by an in vitro budding reaction, GMx33 remains segregated in a matrixlike tail region that sometimes contains Golgin-245. This trans-matrix often links a few vesicles together. Together these data suggest that GMx33 is a member of the trans-Golgi matrix and offer clues regarding the role of the trans-Golgi matrix in sorting and exit from the Golgi.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

