K-ras4B and prenylated proteins lacking "second signals" associate dynamically with cellular membranes
- PMID: 16236799
- PMCID: PMC1345658
- DOI: 10.1091/mbc.e05-05-0408
K-ras4B and prenylated proteins lacking "second signals" associate dynamically with cellular membranes
Abstract
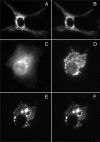
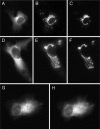
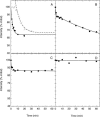
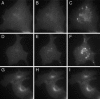
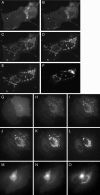
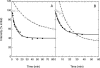
We have used fluorescence microscopy and the technique of rapamycin-regulated protein heterodimerization to examine the dynamics of the subcellular localizations of fluorescent proteins fused to lipid-modified protein sequences and to wild-type and mutated forms of full-length K-ras4B. Singly prenylated or myristoylated fluorescent protein derivatives lacking a "second signal" to direct them to specific subcellular destinations, but incorporating a rapamycin-dependent heterodimerization module, rapidly translocate to mitochondria upon rapamycin addition to bind to a mitochondrial outer membrane protein incorporating a complementary heterodimerization module. Under the same conditions analogous constructs anchored to the plasma membrane by multiply lipid-modified sequences, or by a transmembrane helix, show very slow or no transfer to mitochondria, respectively. Interestingly, however, fluorescent protein constructs incorporating either full-length K-ras4B or its plasma membrane-targeting sequence alone undergo rapamycin-induced transfer from the plasma membrane to mitochondria on a time scale of minutes, demonstrating the rapidly reversible nature of K-ras4B binding to the plasma membrane. The dynamic nature of the plasma membrane targeting of K-ras4B could contribute to K-ras4B function by facilitating redistribution of the protein between subcellular compartments under particular conditions.
Figures

Similar articles
-
Oncogenic and RASopathy-associated K-RAS mutations relieve membrane-dependent occlusion of the effector-binding site.Proc Natl Acad Sci U S A. 2015 May 26;112(21):6625-30. doi: 10.1073/pnas.1419895112. Epub 2015 May 4. Proc Natl Acad Sci U S A. 2015. PMID: 25941399 Free PMC article.
-
Mechanisms of membrane binding of small GTPase K-Ras4B farnesylated hypervariable region.J Biol Chem. 2015 Apr 10;290(15):9465-77. doi: 10.1074/jbc.M114.620724. Epub 2015 Feb 24. J Biol Chem. 2015. PMID: 25713064 Free PMC article.
-
Mutational and biochemical analysis of plasma membrane targeting mediated by the farnesylated, polybasic carboxy terminus of K-ras4B.Biochemistry. 2000 Jul 18;39(28):8298-307. doi: 10.1021/bi000512q. Biochemistry. 2000. PMID: 10889039
-
The Hypervariable Region of K-Ras4B Governs Molecular Recognition and Function.Int J Mol Sci. 2019 Nov 14;20(22):5718. doi: 10.3390/ijms20225718. Int J Mol Sci. 2019. PMID: 31739603 Free PMC article. Review.
-
K-Ras4B/calmodulin/PI3Kα: A promising new adenocarcinoma-specific drug target?Expert Opin Ther Targets. 2016 Jul;20(7):831-42. doi: 10.1517/14728222.2016.1135131. Epub 2016 Feb 12. Expert Opin Ther Targets. 2016. PMID: 26873344 Review.
Cited by
-
Phospholipase D1 regulates lymphocyte adhesion via upregulation of Rap1 at the plasma membrane.Mol Cell Biol. 2009 Jun;29(12):3297-306. doi: 10.1128/MCB.00366-09. Epub 2009 Mar 30. Mol Cell Biol. 2009. PMID: 19332557 Free PMC article.
-
Shuttling and translocation of heterotrimeric G proteins and Ras.Trends Pharmacol Sci. 2009 Jun;30(6):278-86. doi: 10.1016/j.tips.2009.04.001. Epub 2009 May 6. Trends Pharmacol Sci. 2009. PMID: 19427041 Free PMC article. Review.
-
Probing the interactions of the HIV-1 matrix protein-derived polybasic region with lipid bilayers: insights from AFM imaging and force spectroscopy.Eur Biophys J. 2024 Feb;53(1-2):57-67. doi: 10.1007/s00249-023-01697-2. Epub 2024 Jan 3. Eur Biophys J. 2024. PMID: 38172352
-
Cytosolic Ras supports eye development in Drosophila.Mol Cell Biol. 2010 Dec;30(24):5649-57. doi: 10.1128/MCB.00635-10. Epub 2010 Oct 11. Mol Cell Biol. 2010. PMID: 20937772 Free PMC article.
-
Acyl-protein thioesterase 2 catalyzes the deacylation of peripheral membrane-associated GAP-43.PLoS One. 2010 Nov 30;5(11):e15045. doi: 10.1371/journal.pone.0015045. PLoS One. 2010. PMID: 21152083 Free PMC article.
References
-
- Baker, T. L., Booden, M. A., and Buss, J. E. (2000). S-Nitrosocysteine increases palmitate turnover on Ha-Ras in NIH 3T3 cells. J. Biol. Chem. 275, 22037–22047. - PubMed
-
- Baker, T. L., Zheng, H., Walker, J., Coloff, J. L., and Buss, J. E. (2003). Distinct rates of palmitate turnover on membrane-bound cellular and oncogenic Hras. J. Biol. Chem. 278, 19292–19300. - PubMed
-
- Bivona, T. G., Perez De Castro, I., Ahearn, I. M., Grana, T. M., Chiu, V. K., Lockyer, P. J., Cullen, P. J., Pellicer, A., Cox, A. D., and Philips, M. R. (2003). Phospholipase Cγ activates Ras on the Golgi apparatus by means of RasGRP1. Nature 424, 694–698. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

