Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm
- PMID: 16237003
- PMCID: PMC1272965
- DOI: 10.1128/JB.187.21.7193-7203.2005
Competition and coexistence between Streptococcus mutans and Streptococcus sanguinis in the dental biofilm
Abstract
The human mucosal surface is colonized by the indigenous microflora, which normally maintains an ecological balance among different species. Certain environmental or biological factors, however, may trigger disruption of this balance, leading to microbial diseases. In this study, we used two oral bacterial species, Streptococcus mutans and Streptococcus sanguinis (formerly S. sanguis), as a model to probe the possible mechanisms of competition/coexistence between different species which occupy the same ecological niche. We show that the two species engage in a multitude of antagonistic interactions temporally and spatially; occupation of a niche by one species precludes colonization by the other, while simultaneous colonization by both species results in coexistence. Environmental conditions, such as cell density, nutritional availability, and pH, play important roles in determining the outcome of these interactions. Genetic and biochemical analyses reveal that these interspecies interactions are possibly mediated through a well-regulated production of chemicals, such as bacteriocins (produced by S. mutans) and hydrogen peroxide (produced by S. sanguinis). Consistent with the phenotypic characteristics, production of bacteriocins and H2O2 are regulated by environmental conditions, as well as by juxtaposition of the two species. These sophisticated interspecies interactions could play an essential part in balancing competition/coexistence within multispecies microbial communities.
Figures


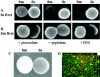


Similar articles
-
Involvement of gshAB in the interspecies competition within oral biofilm.J Dent Res. 2013 Sep;92(9):819-24. doi: 10.1177/0022034513498598. Epub 2013 Jul 19. J Dent Res. 2013. PMID: 23872989
-
Characterization of anti-competitor activities produced by oral bacteria.Methods Mol Biol. 2010;666:151-66. doi: 10.1007/978-1-60761-820-1_11. Methods Mol Biol. 2010. PMID: 20717784
-
Competition and Caries on Enamel of a Dual-Species Biofilm Model with Streptococcus mutans and Streptococcus sanguinis.Appl Environ Microbiol. 2020 Oct 15;86(21):e01262-20. doi: 10.1128/AEM.01262-20. Print 2020 Oct 15. Appl Environ Microbiol. 2020. PMID: 32826216 Free PMC article.
-
The road less traveled - defining molecular commensalism with Streptococcus sanguinis.Mol Oral Microbiol. 2017 Jun;32(3):181-196. doi: 10.1111/omi.12170. Epub 2016 Sep 20. Mol Oral Microbiol. 2017. PMID: 27476770 Free PMC article. Review.
-
[Secondary metabolites from Streptococcus mutans and their ecological roles in dental biofilm].Sheng Wu Gong Cheng Xue Bao. 2017 Sep 25;33(9):1547-1554. doi: 10.13345/j.cjb.170046. Sheng Wu Gong Cheng Xue Bao. 2017. PMID: 28956400 Review. Chinese.
Cited by
-
Role of LytF and AtlS in eDNA release by Streptococcus gordonii.PLoS One. 2013 Apr 24;8(4):e62339. doi: 10.1371/journal.pone.0062339. Print 2013. PLoS One. 2013. PMID: 23638042 Free PMC article.
-
Oral Commensal Streptococci: Gatekeepers of the Oral Cavity.J Bacteriol. 2022 Nov 15;204(11):e0025722. doi: 10.1128/jb.00257-22. Epub 2022 Oct 26. J Bacteriol. 2022. PMID: 36286512 Free PMC article. Review.
-
Transcription of Oxidative Stress Genes Is Directly Activated by SpxA1 and, to a Lesser Extent, by SpxA2 in Streptococcus mutans.J Bacteriol. 2015 Jul;197(13):2160-2170. doi: 10.1128/JB.00118-15. Epub 2015 Apr 20. J Bacteriol. 2015. PMID: 25897032 Free PMC article.
-
The EIIABMan phosphotransferase system permease regulates carbohydrate catabolite repression in Streptococcus gordonii.Appl Environ Microbiol. 2011 Mar;77(6):1957-65. doi: 10.1128/AEM.02385-10. Epub 2011 Jan 14. Appl Environ Microbiol. 2011. PMID: 21239541 Free PMC article.
-
Bacterial interactions in dental biofilm.Virulence. 2011 Sep-Oct;2(5):435-44. doi: 10.4161/viru.2.5.16140. Epub 2011 Sep 1. Virulence. 2011. PMID: 21778817 Free PMC article. Review.
References
-
- Ajdic, D., W. M. McShan, R. E. McLaughlin, G. Savic, J. Chang, M. B. Carson, C. Primeaux, R. Tian, S. Kenton, H. Jia, S. Lin, Y. Qian, S. Li, H. Zhu, F. Najar, H. Lai, J. White, B. A. Roe, and J. J. Ferretti. 2002. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc. Natl. Acad. Sci. USA 99:14434-14439. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

