Type III restriction is alleviated by bacteriophage (RecE) homologous recombination function but enhanced by bacterial (RecBCD) function
- PMID: 16237019
- PMCID: PMC1272966
- DOI: 10.1128/JB.187.21.7362-7373.2005
Type III restriction is alleviated by bacteriophage (RecE) homologous recombination function but enhanced by bacterial (RecBCD) function
Erratum in
- J Bacteriol. 2007 Feb;189(3):1180
Abstract
Previous works have demonstrated that DNA breaks generated by restriction enzymes stimulate, and are repaired by, homologous recombination with an intact, homologous DNA region through the function of lambdoid bacteriophages lambda and Rac. In the present work, we examined the effect of bacteriophage functions, expressed in bacterial cells, on restriction of an infecting tester phage in a simple plaque formation assay. The efficiency of plaque formation on an Escherichia coli host carrying EcoRI, a type II restriction system, is not increased by the presence of Rac prophage-presumably because, under the single-infection conditions of the plaque assay, a broken phage DNA cannot find a homologue with which to recombine. To our surprise, however, we found that the efficiency of plaque formation in the presence of a type III restriction system, EcoP1 or EcoP15, is increased by the bacteriophage-mediated homologous recombination functions recE and recT of Rac prophage. This type III restriction alleviation does not depend on lar on Rac, unlike type I restriction alleviation. On the other hand, bacterial RecBCD-homologous recombination function enhances type III restriction. These results led us to hypothesize that the action of type III restriction enzymes takes place on replicated or replicating DNA in vivo and leaves daughter DNAs with breaks at nonallelic sites, that bacteriophage-mediated homologous recombination reconstitutes an intact DNA from them, and that RecBCD exonuclease blocks this repair by degradation from the restriction breaks.
Figures




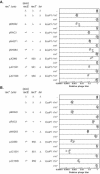



References
-
- Bachi, B., J. Reiser, and V. Pirrotta. 1979. Methylation and cleavage sequences of the EcoP1 restriction-modification enzyme. J. Mol. Biol. 128: 143-163. - PubMed
-
- Bachmann, B. J. 1987. Derivation and genotypes of some mutant derivatives of Escherichia coli K-12, p. 1190-1219. In F. C. Neidhardt, J. L. Ingraham, K. B. Low, B. Magasanik, M. Schaechter, and H. E. Umbarger (ed.), Escherichia coli and Salmonella typhimurium: cellular and molecular biology, vol. 2. American Society for Microbiology, Washington, D.C.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

