Resolvase-in vivo expression technology analysis of the Salmonella enterica serovar Typhimurium PhoP and PmrA regulons in BALB/c mice
- PMID: 16237024
- PMCID: PMC1272988
- DOI: 10.1128/JB.187.21.7407-7416.2005
Resolvase-in vivo expression technology analysis of the Salmonella enterica serovar Typhimurium PhoP and PmrA regulons in BALB/c mice
Abstract
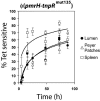
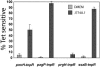
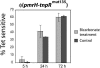
Salmonella enterica modulates resistance to antimicrobial peptides in part via covalent modifications of the lipopolysaccharide (LPS). The two-component systems PhoP/PhoQ and PmrA/PmrB are activated during infection and regulate several genes involved in LPS modifications by responding to signals such as pH, iron, magnesium, and antimicrobial peptides. A recombination-based in vivo expression technology approach was adopted to analyze the spatial-temporal patterns of in vivo expression of genes of the PhoP and PmrA regulons and to identify the in vivo signals modulating their transcription. In vitro, we showed PhoP- and/or PmrA-dependent induction of pmrH (LPS aminoarabinose modification operon) by acidic pH, low levels of magnesium, or high levels of Fe(III). Upregulation in cultured J774A.1 macrophages was shown for pmrH, pagP (LPS palmitate addition), and ssaB (pathogenicity island II secretion) but not for prgH (pathogenicity island I secretion). Increased levels of pmrH, phoP, and prgH transcription but not ssaB were observed in bacteria isolated from the lumen of the distal ileum. Bacteria isolated from spleens of orally inoculated mice showed no further induction of prgH but had the highest expression of pmrH, pagP, and ssaB. In vivo induction of pmrH was fully dependent on pmrA and phoP, and buffering stomach acidity, iron chelation, or low-iron diets did not affect the expression of pmrH in the intestinal lumen. The observation of pmrH and pagP expression in the intestine refutes the paradigm of PhoP/PhoQ and PmrA/PmrB in vivo expression as solely intracellularly induced and supports previous data demonstrating peroral virulence attenuation of pmrH mutants.
Figures




Similar articles
-
Identification and functional analysis of Salmonella enterica serovar Typhimurium PmrA-regulated genes.FEMS Immunol Med Microbiol. 2005 Feb 1;43(2):249-58. doi: 10.1016/j.femsim.2004.08.007. FEMS Immunol Med Microbiol. 2005. PMID: 15681155
-
Genetic and functional analysis of a PmrA-PmrB-regulated locus necessary for lipopolysaccharide modification, antimicrobial peptide resistance, and oral virulence of Salmonella enterica serovar typhimurium.Infect Immun. 2000 Nov;68(11):6139-46. doi: 10.1128/IAI.68.11.6139-6146.2000. Infect Immun. 2000. PMID: 11035717 Free PMC article.
-
Identification and genetic characterization of PmrA-regulated genes and genes involved in polymyxin B resistance in Salmonella enterica serovar typhimurium.Infect Immun. 2002 Dec;70(12):6770-8. doi: 10.1128/IAI.70.12.6770-6778.2002. Infect Immun. 2002. PMID: 12438352 Free PMC article.
-
The biology of the PmrA/PmrB two-component system: the major regulator of lipopolysaccharide modifications.Annu Rev Microbiol. 2013;67:83-112. doi: 10.1146/annurev-micro-092412-155751. Epub 2013 Jun 17. Annu Rev Microbiol. 2013. PMID: 23799815 Free PMC article. Review.
-
The PhoQ/PhoP regulatory network of Salmonella enterica.Adv Exp Med Biol. 2008;631:7-21. doi: 10.1007/978-0-387-78885-2_2. Adv Exp Med Biol. 2008. PMID: 18792679 Review.
Cited by
-
Role of lipid A acylation in Yersinia enterocolitica virulence.Infect Immun. 2010 Jun;78(6):2768-81. doi: 10.1128/IAI.01417-09. Epub 2010 Apr 12. Infect Immun. 2010. PMID: 20385763 Free PMC article.
-
Contribution of the Salmonella enterica KdgR Regulon to Persistence of the Pathogen in Vegetable Soft Rots.Appl Environ Microbiol. 2015 Dec 18;82(4):1353-1360. doi: 10.1128/AEM.03355-15. Print 2016 Feb 15. Appl Environ Microbiol. 2015. PMID: 26682862 Free PMC article.
-
Palmitoylation state impacts induction of innate and acquired immunity by the Salmonella enterica serovar typhimurium msbB mutant.Infect Immun. 2011 Dec;79(12):5027-38. doi: 10.1128/IAI.05524-11. Epub 2011 Sep 19. Infect Immun. 2011. PMID: 21930761 Free PMC article.
-
Impact of the Resistance Responses to Stress Conditions Encountered in Food and Food Processing Environments on the Virulence and Growth Fitness of Non-Typhoidal Salmonellae.Foods. 2021 Mar 14;10(3):617. doi: 10.3390/foods10030617. Foods. 2021. PMID: 33799446 Free PMC article. Review.
-
Substrate structure-activity relationship reveals a limited lipopolysaccharide chemotype range for intestinal alkaline phosphatase.J Biol Chem. 2019 Dec 13;294(50):19405-19423. doi: 10.1074/jbc.RA119.010836. Epub 2019 Nov 8. J Biol Chem. 2019. PMID: 31704704 Free PMC article.
References
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1987. Current protocols in molecular biology, vol. 1. John Wiley & Sons, New York, N.Y.
-
- Bader, M. W., W. W. Navarre, W. Shiau, H. Nikaido, J. G. Frye, M. McClelland, F. C. Fang, and S. I. Miller. 2003. Regulation of Salmonella typhimurium virulence gene expression by cationic antimicrobial peptides. Mol. Microbiol. 50:219-230. - PubMed
-
- Brogden, K. A., M. Ackermann, P. B. McCray, Jr., and B. F. Tack. 2003. Antimicrobial peptides in animals and their role in host defences. Int. J. Antimicrob. Agents 22:465-478. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

